
Mesenteric cystic lymphatic malformation: a rare case report and review of the literature
Highlight box
Key findings
• Although lymphatic malformation (LM) appears cystic, its imaging features are still complex and often lead to misdiagnosis.
What is known and what is new?
• It is well known that LM is extremely rare and lack specific clinical manifestations, making their accurate diagnosis challenging.
• After reviewing the relevant literature, it was found that the combination of imaging examination and endoscopic technology could significantly improve the diagnostic rate of this disease.
What is the implication, and what should change now?
• Through the above examination methods, the size and location of LM can be obtained and classified according to the size of the cystic part, so as to develop treatment strategies for distinct types of LM.
Introduction
Lymphatic malformation (LM) is a rare benign congenital malformation of the lymphatic system (1,2). Most LMs typically occur in the head and neck region (3), whereas the disease can present at any age (4), although it is very rare in adults (5). LM is clinically challenging to diagnose due to atypical clinical manifestations or lack of recognition of the disease, so early diagnosis is essential for treatment modalities and prognostic effects. Imaging examination can assist in the diagnosis and differential diagnosis of LM. This paper reports a case of LM of the sigmoid colon in an adult female, and reviews the relevant literature to provide effective clues for the diagnosis and treatment of LM of the sigmoid colon. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-143/rc).
Case presentation
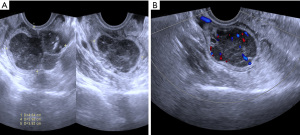
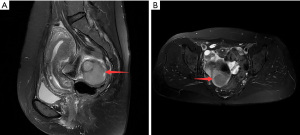
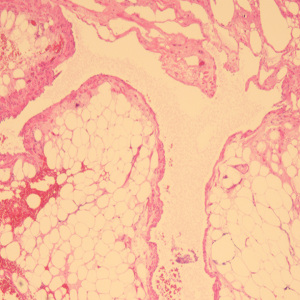
A 49-year-old female patient underwent a physical examination at the First People’s Hospital of Linhai City one month ago. Transvaginal ultrasound (Figure 1A,1B) revealed a hypoechoic mass measuring 46 mm × 30 mm × 39 mm, characterized by a well-defined border, thick wall, irregular shape, heterogeneous internal echo, intense echogenicity, and blood flow signal in the posterior region of the uterus. The mass was suspected to be of intestinal origin. To further investigate this condition, the patient was admitted to our hospital with the chief complaint of pelvic mass detected for 1 month. Contrast-enhanced pelvic magnetic resonance imaging (MRI) (Figure 2A,2B) revealed a solid mass located posterior to the uterus and anterior to the sigmoid colon on the right side of the pelvis. The tumor exhibited isointensity on T1-weighted imaging, slight hyperintensity on T2-weighted imaging with fat suppression, hyperintensity on diffusion-weighted imaging, and hypointensity on apparent diffusion coefficient mapping. Furthermore, the mass displayed well-defined margins and lobulated characteristics. It demonstrated close association with the sigmoid colon without infiltration into adjacent adipose tissue. Contrast-enhanced scan demonstrated marked heterogeneous enhancement suggestive of a stromal tumor. Abdominal palpation revealed a flat and soft abdomen with no evidence of hepatosplenomegaly or generalized abdominal tenderness. Following the exclusion of surgical contraindications, laparoscopic sigmoid mesocolic cyst fenestration and intestinal adhesiolysis were performed under general anesthesia. The surgical procedure revealed a cyst measuring approximately 60 mm × 40 mm × 30 mm in size at the mesentery of the sigmoid colon, located posterior to the uterus. This cyst was effectively managed through fenestration and drainage, revealing multiple internal septations. Tissue samples obtained from the cyst wall exhibited characteristics consistent with a mesangial cyst of the sigmoid colon, accompanied by foam cell aggregation without any malignant changes. Postoperative immunohistochemical pathological (Figure 3) analysis further confirmed the diagnosis of sigmoid lymphangioma. The patient was examined by ultrasound 3 months after surgery and did not observe any signs of recurrence. We plan to follow the patient continuously for one year.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Intestinal LM in adults is an exceedingly rare benign disease (5), and its pathogenesis arises from the presence of cystic regions with local lymphatic stasis due to developmental anomalies in regional lymphatic drainage, rather than a true neoplastic growth (1). The disease mainly occurs in people between the ages of 20 and 50 years, without any apparent gender predilection (6). The age distribution supports the notion that LM represents a congenital malformation often diagnosed belatedly owing to its slow growth or asymptomatic nature. Seventy percent of LMs predominantly manifest in the head and neck region, often leading to dysphagia and discomfort due to potential bleeding. Approximately 25% of cases occur in the chest wall and limbs, while a minority (5%) are found within internal organs, primarily located in the mesentery, omentum, and mesocolon (4,7). Notably, studies have reported that abdominal LM is most frequently observed within the small intestine among 48 adults (8), with approximately 25% occurring specifically in the duodenum followed by involvement of the small intestine (19%). Conversely, occurrences within the mesentery are relatively rare (10%), with only one documented case identified in the sigmoid colon. The classification of LM is based on the microscopic evaluation of the size of the lymphatic space, resulting in three subtypes: lymphatic capillary type, cavernous type, and cystic type, as observed through clinicopathological analysis (9,10). The disorder is characterized by a thin and irregular cyst wall with multiple internal septa, composed of endothelial cells, smooth muscle cells, foam cells, and lymphoid tissue (8). The location, size, and characteristics of the disease play a crucial role in its clinical presentation. When the mass is small, there are typically no apparent subjective symptoms, and most cases are incidentally detected during physical examination. As the mass gradually enlarges and exerts pressure on adjacent structures, it may elicit a sensation of dragging, accompanied by a range of associated manifestations such as nausea, vomiting, hematuria, and abdominal discomfort (9,11). It is worth noting that no cases of malignant transformation have been reported in this significant abnormality. Abdominal pain and distension are the prevailing clinical manifestations. Despite being a benign lesion, certain studies have indicated potential for more severe complications such as intestinal wall rupture, secondary infection, gastrointestinal bleeding, volvulus or intestinal obstruction (12,13). Among them, intestinal obstruction is the most prevalent complication (14). Enlargement of lymphangioma in the mesentery or intestine results in mechanical incomplete intestinal obstruction, characterized by clinical manifestations such as cessation of flatus and defecation. Moreover, this condition represents a primary indication for exploratory laparotomy.
Currently, ultrasound and computed tomography (CT) examinations have emerged as the primary modalities for initial LM screening due to their non-invasive nature and straightforward implementation. Moreover, they can be employed for precise localization diagnosis of LM. The typical ultrasound characteristics of intestinal LM include cystic or cystic-solid appearance with well-defined boundaries and multiple thin or thick septations. Color Doppler imaging reveals the absence of blood flow signal or color blood flow signal in both the periphery and interior (2,6,7). However, it should be noted that sound transmission may be compromised in the cystic portion due to hemorrhage or fibrin deposition (15). Li et al. classified according to the size of the cyst diameter, the macrocystic (cysts >1 cm in diameter), microcystic (individual cysts <1 cm in diameter), and mixed types were classified according to the cyst diameter (16). The abdominal CT examination has been widely recognized as a gold standard for diagnosing LM, enabling the evaluation of tumor density and providing comprehensive information regarding affected organs and their contents. The CT examination reveals well-defined borders with low density or heterogeneous cystic or solid components, displaying unilocular or multilocular structures that exhibit either no enhancement or only mild enhancement of the septum and cyst wall following contrast injection. It has been suggested that MRI can serve as an adjunctive tool for discerning the potential origin and positional relationships between lesions and adjacent structures. MRI revealed a multilobular septal configuration of the lesion, exhibiting hypointensity on T1-weighted images and hyperintensity on T2-weighted images. In cases of pure type LM, contrast material administration via intravenous route demonstrated enhancement of the cyst wall and septum (17), consistent with the presented findings. Based on the imaging findings of the aforementioned examination methods, although it is possible to determine the internal echo, size, shape, location, and extent of invasion of the lesion, qualitative diagnosis remains challenging. In some instances, there may be missed diagnoses, particularly for lesions situated in the intestine and mesentery. Endoscopic ultrasound (EUS) plays a pivotal role in diagnosing LM by providing precise information regarding tumor origin layer (18,19). The combined utilization of colonoscopy enhances diagnostic accuracy.
In terms of treatment, regular follow-up is recommended for patients with small LM and no symptoms. Based on the findings from Chen et al. (20), surgical resection is advised when the median size of LM exceeds 12.5 cm and there are clinical symptoms and/or complications present, whereas simple follow-up is not recommended. The recurrence rate can be as high as 40% (12), with recurrence closely associated with the location of LM. To minimize the risk of tumor recurrence, it is crucial to perform a comprehensive resection of all abnormal tissue and ligate the surrounding lymphatic vessels during surgery (7). In cases where the tumor infiltrates adjacent bowel, major branching vessels, or other organs, simultaneous resection of the affected organ along with its free margins is necessary to effectively reduce the likelihood of recurrence (12). Nevertheless, non-invasive treatment alternatives such as aspiration and percutaneous injection of sclerosing agents are also viable options. Thiam et al. (21) proposed that in the management of complex or unresectable masses, cysts can be punctured using a fine needle for content evacuation, with or without concurrent administration of sclerosing agents. However, Zobel et al. (22) highlighted the limited efficacy of percutaneous injection sclerotherapy in patients with microcystic LM.
Conclusions
After careful consideration, it is evident that the clinical manifestations of LM lack specificity, leading to potential instances of missed diagnosis and misdiagnosis. The diverse presentation patterns observed in LM are influenced by factors such as lesion size and location. By leveraging the imaging characteristics of intestinal LM alongside endoscopic ultrasonography, clinicians can enhance the diagnostic accuracy for this disease while formulating comprehensive and effective treatment strategies, ultimately improving patient prognosis.
Acknowledgments
Funding: The present study was supported by grants from the National Natural Science Foundation of China (grant No. 81974470; grant No. 82272004) and the Natural Science Foundation of Zhejiang Province (grant No. LY18H180001).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-143/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-143/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-143/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hoang VT, Nguyen MD, Van HAT, et al. Review of diagnosis, differential diagnosis, and management of retroperitoneal lymphangioma. Jpn J Radiol 2023;41:283-301. [PubMed]
- Maghrebi H, Yakoubi C, Beji H, et al. Intra-abdominal cystic lymphangioma in adults: A case series of 32 patients and literature review. Ann Med Surg (Lond) 2022;81:104460. [Crossref] [PubMed]
- Shayesteh S, Salimian KJ, Fouladi DF, et al. Intra-abdominal lymphangioma: A case report. Radiol Case Rep 2021;16:123-7. [Crossref] [PubMed]
- Li Y, Wang Q, Kan G, et al. Renal lymphangiomatosis: literature analysis on research progress and presentation of four cases. Quant Imaging Med Surg 2023;13:518-28. [Crossref] [PubMed]
- Mabrouk MY, Magouri O, Madani A, et al. Mesenteric cystic lymphangioma in an adult: An unusual case report. Ann Med Surg (Lond) 2022;78:103917. [Crossref] [PubMed]
- Chin CC, Shiau J, Luo CW, et al. Lymphangioma of small bowel in adults: A rare cause of abdominal symptoms. Asian J Surg 2023;46:863-7. [Crossref] [PubMed]
- Abebe DM, Nureta TH, Gima T. A rare case of huge intra-abdominal cystic lymphangioma arising from rectovesical pouch; a case report. Int J Surg Case Rep 2023;106:108275. [Crossref] [PubMed]
- Mede A, Chotai PN, Huh WJ, et al. Intra-abdominal Cystic Lymphangiomas: The Vanderbilt Experience. J Surg Res 2023;285:197-204. [Crossref] [PubMed]
- Radhouane A, Mayada S, Khaled N. Lymphangioma of the ovary: etiology and management. Eur J Obstet Gynecol Reprod Biol 2016;203:342-3. [Crossref] [PubMed]
- Chung JC, Song OP. Cystic lymphangioma of the jejunal mesentery presenting with acute abdomen in an adult. Can J Surg 2009;52:E286-8. [PubMed]
- Perez A, Perez MEC, Yuga AC, et al. Splenic lymphangioma in adulthood: A case report. Int J Surg Case Rep 2020;67:250-3. [Crossref] [PubMed]
- Azimi B, Bagherian Lemraski S, Kouchak Hosseini SP, et al. Small bowel volvulus and mesenteric ischemia induced by mesenteric cystic lymphangioma in an adult and literature review; a case report. Int J Surg Case Rep 2023;105:108083. [Crossref] [PubMed]
- Rani DV, Srilakshmi R, Malathi S, et al. Unusual presentation of a retroperitoneal lymphangioma. Indian J Pediatr 2006;73:617-8. [Crossref] [PubMed]
- Xiao J, Shao Y, Zhu S, et al. Characteristics of adult abdominal cystic Lymphangioma: a single-center Chinese cohort of 12 cases. BMC Gastroenterol 2020;20:244. [Crossref] [PubMed]
- Pham HD, Nguyen TA, Doan TG, et al. Lymphangioma of Colon Presenting as an Intramural Tumor. Int Med Case Rep J 2022;15:361-6. [Crossref] [PubMed]
- Li J, Zhong W, Geng X, et al. Ultrasonographic diagnosis, classification, and treatment of cervical lymphatic malformation in paediatric patients: a retrospective study. BMC Pediatr 2020;20:441. [Crossref] [PubMed]
- Aslan A, Büyükkaya R, Tan S, et al. Efficacy of ultrasonography in lymphatic malformations: diagnosis, treatment and follow-up: a case report. Med Ultrason 2013;15:244-6. [Crossref] [PubMed]
- Bhutani MS, Annangi S, Koduru P, et al. Diagnosis of cystic lymphangioma of the colon by endoscopic ultrasound: Biopsy is not needed! Endosc Ultrasound 2016;5:335-8. [Crossref] [PubMed]
- He G, Wang J, Chen B, et al. Feasibility of endoscopic submucosal dissection for upper gastrointestinal submucosal tumors treatment and value of endoscopic ultrasonography in pre-operation assess and post-operation follow-up: a prospective study of 224 cases in a single medical center. Surg Endosc 2016;30:4206-13. [Crossref] [PubMed]
- Chen KY, Wu CH, Chen KH, et al. Abdominal cystic lymphangioma presenting fever in an adult. Asian J Surg 2023;46:2899-900. [Crossref] [PubMed]
- Thiam O, Faye PM, Niasse A, et al. Cystic mesenteric lymphangioma: A case report. Int J Surg Case Rep 2019;61:318-21. [Crossref] [PubMed]
- Zobel MJ, Nowicki D, Gomez G, et al. Management of cervicofacial lymphatic malformations requires a multidisciplinary approach. J Pediatr Surg 2021;56:1062-7. [Crossref] [PubMed]
Cite this article as: Li YY, Wang Q, Zhu J. Mesenteric cystic lymphatic malformation: a rare case report and review of the literature. AME Case Rep 2024;8:23.


