Urinothorax and pseudo-azotemia following total abdominal hysterectomy: a case report
Highlight box
Key findings
• Urinothorax is rare occurrence secondary to a commonly performed gynecologic procedure.
• Presenting symptoms include fever, dyspnea, chest pain, abdominal pain, and decreased urine output.
• Pleural fluid analysis generally shows a pleural fluid/serum creatinine ratio >1.
• Pseudo-azotemia can cause marked elevations in serum creatinine which promptly improves after therapeutic intervention is made.
• Treatment is aimed at correcting underlying genitourinary (GU) pathology.
What is known and what is new?
• Urinothorax is an uncommon and underdiagnosed thoracic complication caused by iatrogenic or traumatic GU injury, or obstructive uropathy.
• Pseudo-azotemia is a process causing marked elevations in serum creatinine in the setting of urinothorax explained by reabsorption of creatinine through the peritoneal membrane or peritoneo-pleural lymphatic system.
What is the implication, and what should change now?
• Physicians across all specialties should be aware of potential complications related to various urologic and gynecologic procedures including respiratory compromise by means of a urinothorax.
Introduction
Urinothorax is a rare cause of pleural effusion characterized by the accumulation of urine in the pleural space arising as a consequence of trauma, obstruction, or iatrogenic causes (1,2). From its initial documentation in 1968 until 2016, a total of 88 patients afflicted with urinothorax were reported. Notably, male patients accounted for a majority (61.4%) of cases, with a median age of 45 years (2). Trauma was implicated as the causative factor in 76% of cases, while the remaining cases were attributable to obstructive uropathy. Herein, we report a case of a female in her early 50s who presented with abdominal pain and vomiting after recently undergoing a total abdominal hysterectomy. She subsequently developed shortness of breath and was diagnosed with right-sided pleural effusion of extravascular origin (PEEVO). We presented this article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-146/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
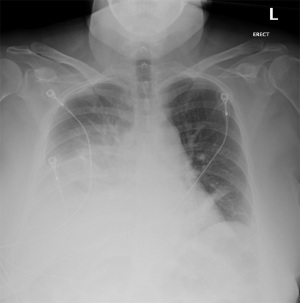
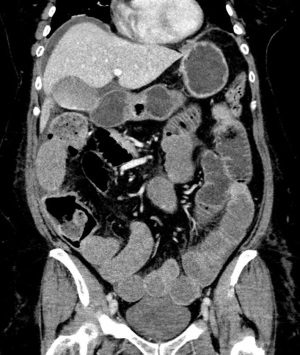
Our patient was a 52-year-old female with a past medical history of hypertension, dyslipidemia, and endometriosis who presented to the emergency department with hyperemesis and intermittent sharp lower abdominal pain. Six days prior to presentation, she underwent a total abdominal hysterectomy at another hospital for endometriosis. Initial laboratory results with reference range showed white blood cell count of 13.9 K/mcL (4.5–11 K/mcL), platelets 638 K/mcL (140–440 K/mcL), Na+ 128 mmol/L (135–145 mmol/L), K+ 3.1 mmol/L (3.5–5.5 mmol/L), blood urea nitrogen (BUN) 23.9 mg/dL (9–27 mg/dL), and creatinine (Cr) 1.9 mg/dL (0.6–1.5 mg/dL). Abdominal ultrasound was performed for evaluation of acute kidney injury and pyuria which revealed small ascites along with small pleural effusion. Computed tomography (CT) of the abdomen revealed a small amount of ascites attributed to postoperative fluid collection, bibasilar lung atelectasis with no signs of pleural effusion as well as distended small bowel loops with scattered air-fluid levels likely related to postoperative ileus (Figure 1). Postoperative ileus was managed expectantly with anti-emetics, prokinetics, ambulation, avoidance of narcotics and maintenance fluids with electrolyte supplementation. CT scan did not suggest a mechanical obstruction. Surgery was consulted and recommended non-operative management. Over the next two days, she began to experience progressive shortness of breath, tachycardia, oliguria, and elevation of BUN and Cr to 33.4 and 3.2 mg/dL, respectively. A large right-sided pleural effusion was suspected on physical examination and confirmed on chest X-ray (Figure 2); thoracentesis yielded 2 L of straw-colored fluid, which was sent for analysis. Follow-up chest X-ray demonstrated a decrease in effusion size. Concurrently, urine output was progressively decreasing, and Cr levels trended up to 3.9 mg/dL. Despite our interventions, the patient’s dyspnea persisted. A repeat chest X-ray revealed re-accumulation of fluid, leading to prompt placement of a right-sided chest tube, which yielded 3 L of straw-colored fluid. Pleural fluid analysis showed transudative fluid with pH 7.5, Cr 5.8 mg/dL, lactate dehydrogenase (LDH) 172 unit/L, and total protein 1,170 mg/dL. Pleural fluid/serum Cr ratio was 1.4. Differential diagnoses that were effectively excluded in this case include, gastric and esophageal perforation and, pleural effusion secondary to ascites, which was substantiated by presence of minimal ascites and no pleural effusion noted on CT scan of the abdomen done at the time of admission. Furthermore, the prospect of complications associated with pleural endometriosis, including hemothorax, was effectively ruled out as such complications are predominantly catamenial and inconsistent with our pleural fluid analysis.
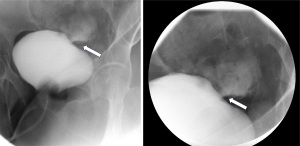
The decision to place a Foley catheter was made, and there was a prompt improvement in serum Cr to 0.6 mg/dL within the next 24 hours. Due to a high degree of clinical suspicion, urology was consulted, and an X-ray cystogram (Figure 3) was done, which confirmed a <1 cm leak in the dome of the urinary bladder, thereby confirming the diagnosis of urinothorax. The chest tube was removed once the output was less than 100 cc per 24 hours, and the patient was discharged with a Foley catheter to allow for the healing of the bladder laceration.
Discussion
Pleural effusion is a common condition, affecting approximately 1.5 million individuals annually in the United States alone (3). Effusions may be classified as either transudative, which commonly arise due to imbalances in hydrostatic and oncotic pressures, or exudative, which frequently result from infection, inflammation, malignancy, lymphatic abnormalities, and other causes. Less common types of transudative and exudative pleural effusions include those of extravascular origin. A PEEVO is one that does not originate from the pulmonary vasculature. Due to the infrequency of PEEVOs, a high index of suspicion is required to prompt appropriate diagnosis and management. PEEVO with exudative etiologies encompasses causes such as esophageal or gastric perforation, enteral feeding tube migration, pancreaticopleural fistula, bilothorax, chylothorax, pleural endometriosis and cholesterol effusion. In contrast, transudative causes consist of peritoneal dialysate, extravascular migration of a central venous catheter, ventriculopleural shunts, glycinothorax, hepatic hydrothorax, and urinothorax (4).
Urinothorax refers to the presence of urine in the pleural space. The incidence of urinothorax is yet to be determined in view of the absence of clear diagnostic criteria, compounded by the lack of knowledge and poor recognition of this condition among healthcare providers. The occurrence of urinothorax has been observed to follow a bimodal age distribution, with a higher frequency in the age groups of 41–50 and 61–70 years. Additionally, it was more likely to have a unilateral right-sided presentation and occupy over two-thirds of the hemithorax.
Presenting symptoms of urinothorax may vary. According to a 2017 systematic review, patients with this condition most commonly presented with fever, dyspnea, chest pain, abdominal pain, and decreased urine output (2). Various conditions have been documented as causes of urinothorax, including obstructive uropathy with hydronephrosis, intra-abdominal compression from a gravid uterus, polycystic renal disease, bladder malignancy, and trauma such as extracorporeal lithotripsy, blunt trauma, and surgical injury caused by hysterectomy, nephrolithotomy, and nephrostomy tube placement, among others (2,5). The occurrence of damage to the lower urinary tract during a hysterectomy can vary between 0.13% and 3.6% for bladder injuries and between 0.1% and 1.8% for ureteral injuries (6). The pathophysiology behind the translocation of urine from the genitourinary tract into the thorax is poorly understood; however, it is believed to be caused by transmigration through the diaphragmatic pores propelled by a pressure gradient, or by drainage via the peritoneo-pleural lymphatic system (1).
Only three cases of urinothorax have been reported in the literature as complications of abdominal hysterectomy at the time of this writing. Table 1 displays all four cases (including this one) and outlines the patients’ demographics, presentations, and interventions. Symptomatic presentations were similar, including postoperative dyspnea. Thoracentesis was done, and pleural fluid characteristics among these patients were similarly described as transudative, with a pH between 7.2–8.0. It is worth mentioning that a strict anaerobic environment must be maintained when sending pleural fluid samples for testing and fluid analysis may be unreliable (especially pH) if this is not followed or if processing of sample is delayed. Additionally, similar cases noted a pleural fluid/serum Cr ratio >1 and elevations in serum Cr were noted in two out of three cases, likely explained by reabsorption of Cr through the peritoneal membrane or peritoneo-pleural lymphatics causing pseudo-azotemia (7). These cases were resolved by relieving the urinary obstruction, draining the urinoma, or repairing the bladder injury (7-9).
Table 1
| Patient factors | Goto et al. 2010, (7) | Amro et al. 2009, (8) | Chan et al. 2016, (9) | Mikhail et al. 2023 |
|---|---|---|---|---|
| Age, years | 37 | Premenopausal | 51 | 52 |
| Sex | Female | Female | Female | Female |
| Procedure | TAH | TAH-LSO | Laparoscopic TAH-BSO | TAH-BSO |
| Clinical presentation | Postoperative abdominal distention with shifting dullness and diffuse tenderness | Postoperative sudden onset shortness of breath | Not reported | Postoperative abdominal distention and shortness of breath |
| Organ injured | Bladder | Bladder | Ureter | Bladder |
| Pleural fluid-to-serum creatinine ratio | Not reported | 9.9 | 1.02 | 1.4 |
| Intervention | Perforation closed surgically | Repair of urinary bladder rent | Percutaneous urine drainage | Foley catheter placement |
TAH, total abdominal hysterectomy; LSO, left salpingo-oophorectomy; BSO, bilateral salpingo-oophorectomy.
Although urinothorax is a rare occurrence of a commonly performed gynecologic procedure, it is imperative that physicians be prepared to effectively address such medical complications. Careful review of the literature revealed that prophylactic placement of ureteral catheters has several merits, including the reduction of ureteral injury risk, a decrease in operative duration and mitigation of intraoperative hemorrhage. Considering these potential benefits, prophylactic ureteral catheters may serve as a viable preoperative strategy prior to gynecologic surgeries particularly when dealing with pelvic adhesions (10).
Conclusions
This case highlights the importance for physicians across all specialties to be aware of potential complications related to various urologic and gynecologic procedures, including respiratory compromise by means of a urinothorax. Physicians should consider PEEVO within their differential when intrathoracic causes of pleural effusion have been excluded. Prompt recognition and intervention can improve outcomes by decreasing respiratory complications and shorten or avoid intensive care unit stays. The documentation of additional cases would allow for a better understanding of the development, varied presentations, and time course of urinothorax, resulting in improvements in its diagnosis and management.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-146/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-146/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-146/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Austin A, Jogani SN, Brasher PB, et al. The Urinothorax: A Comprehensive Review With Case Series. Am J Med Sci 2017;354:44-53. [Crossref] [PubMed]
- Toubes ME, Lama A, Ferreiro L, et al. Urinothorax: a systematic review. J Thorac Dis 2017;9:1209-18. [Crossref] [PubMed]
- Krishna R, Antoine MH, Rudrappa M. Pleural Effusion. In StatPearls. StatPearls Publishing; 2023.
- Sahn SA. Pleural effusions of extravascular origin. Clin Chest Med 2006;27:285-308. [Crossref] [PubMed]
- Ranjan V, Agrawal S, Chipde SS, et al. Urinothorax: A path, less travelled: Case report and review of literature. J Nat Sci Biol Med 2015;6:213-6. [Crossref] [PubMed]
- Mamik MM, Antosh D, White DE, et al. Risk factors for lower urinary tract injury at the time of hysterectomy for benign reasons. Int Urogynecol J 2014;25:1031-6. [Crossref] [PubMed]
- Goto S, Yamadori M, Igaki N, et al. Pseudo-azotaemia due to intraperitoneal urine leakage: a report of two cases. NDT Plus 2010;3:474-6. [PubMed]
- Amro O, Webb-Smith F, Sunderji S. Urinothorax: a rare complication of total abdominal hysterectomy. Obstet Gynecol 2009;114:482-4. [Crossref] [PubMed]
- Chan DM, Cheung VY. Urinothorax as an early sign of urinary tract injury following total laparoscopic hysterectomy. Int J Gynaecol Obstet 2016;134:102-3. [Crossref] [PubMed]
- Feng D, Tang Y, Yang Y, et al. Does prophylactic ureteral catheter placement offer any advantage for laparoscopic gynecological surgery? A urologist’ perspective from a systematic review and meta-analysis. Transl Androl Urol 2020;9:2262-9. [Crossref] [PubMed]
Cite this article as: Mikhail E, Seri A, Ralston K, Valvani A, Kalynych Z. Urinothorax and pseudo-azotemia following total abdominal hysterectomy: a case report. AME Case Rep 2024;8:25.