Myocardial ischemia in the setting of extrinsic coronary compression: two case reports
Highlight box
Key findings
• Extrinsic compression of the coronaries secondary to pulmonary artery dilation or mechanical obstruction from nearby chest tubes or drain can cause ischemia and lead to catastrophic consequences.
What is known and what is new?
• Limited management guidelines are available given rarity of this presentation.
• Diagnosis with emergent coronary angiography and intravascular ultrasound should be performed if extrinsic compression is suspected, and treatment with percutaneous coronary intervention or adjusting nearby tubes/drains results in favorable outcomes.
What is the implication, and what should change now?
• High clinical suspicion is warranted for proper diagnosis and treatment.
Introduction
In the United States, one in four deaths occurs from cardiovascular diseases of which coronary artery disease (CAD) contributes to a significant portion (1). CAD is commonly caused by coronary artery atherosclerosis that leads to plaque buildup, causing narrowing of the vessels which ultimately can lead to decreased blood flow or complete absence of flow. Inadequate supply of blood and oxygen to the myocardium causes myocardial infarction. Atherosclerosis is the most common cause of acute myocardial infarction (AMI); however, many infrequent causes of AMI have been reported (2). Extrinsic compression of the coronary artery as a cause of myocardial ischemia is unusual and infrequently seen yet can lead to catastrophic clinical consequences. Here, we present two rare causes of extrinsic compression of coronary blood supply from our institution that led to cardiac arrest and discuss their clinical presentation, diagnostic modalities, and management options, along with a brief review of the available literature to allow for prompt recognition and management by clinicians. We present both cases in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-111/rc).
Case presentation
Case 1
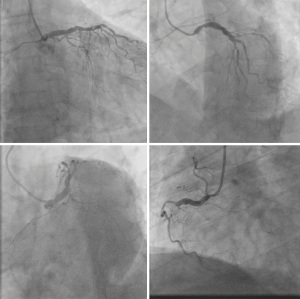
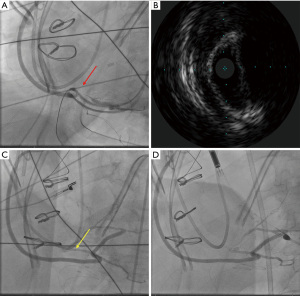
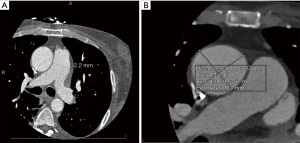
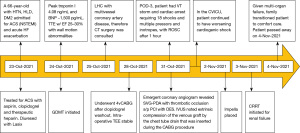
A 66-year-old male with a history of hypertension, hyperlipidemia, and diabetes mellitus presented with worsening chest pain and dyspnea on exertion and was admitted for management of acute congestive heart failure exacerbation and non-ST elevation myocardial infarction (NSTEMI) with a peak troponin I of 4.08 ng/mL. Patient was loaded with aspirin, clopidogrel and was started on therapeutic heparin. Transthoracic echocardiogram demonstrated an ejection fraction of 26% with wall motion abnormalities. The patient underwent coronary angiography which revealed severe multivessel CAD (Figure 1). The patient had an urgent four-vessel coronary artery bypass graft (CABG) with left internal mammary artery to left anterior descending artery, sequenced reversed saphenous vein graft (SVG) to the obtuse marginal artery, and posterior descending artery, reversed SVG to ramus intermedius artery. Total bypass time was 92 minutes and total cross-clamp time was 73 minutes. Intra-operative transesophageal echocardiogram was stable with no new wall motion abnormality noted. His initial post-operative course was uncomplicated; however, on the post-operative day three the patient subsequently developed pulseless ventricular tachycardia arrest. The resuscitation efforts lasted over an hour, including delivery of eighteen shocks for refractory ventricular tachycardia. Return of spontaneous circulation (ROSC) was ultimately achieved; however, patient was on multiple pressors and inotropes at this time. The patient was taken for an emergent coronary angiography which revealed an acute thrombotic occlusion of the saphenous venous graft to the right posterior descending artery (Figure 2A). The lesion was hazy and right above the lesion was an echo-dense structure which was later identified to be a drain tube. Intravascular ultrasound (IVUS) revealed extrinsic compression of the venous graft by the 19 mm Jackson-Pratt (JP) drain that was inserted during his CABG operation (Figure 2B). The drain tube was adjusted, and given the severity of the patient’s cardiogenic shock, the lesion was treated with a Medtronic Resolute Onyx 3.5 mm × 26 mm drug eluting stent (DES) with the restoration of the thrombolysis in myocardial infarction (TIMI) III flow noted (Figure 2C). Immediately following stent placement, patient’s hemodynamics significantly improved. His course was further complicated by worsening cardiogenic shock and multiorgan failure requiring mechanical support. During the placement of mechanical support, patency of SVG was noted on the repeat coronary angiogram (Figure 2D). Ultimately, the patient was transitioned to comfort care per the family wishes and he passed.
Case 2
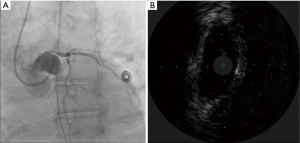
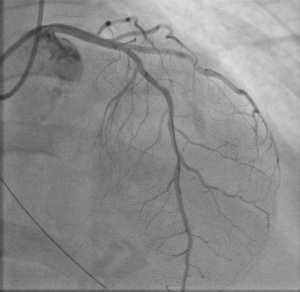
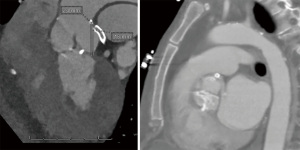
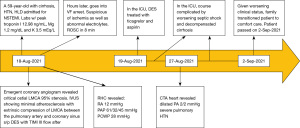
A 59-year-old female with a history of cirrhosis, hypertension, and hyperlipidemia presented with shortness of breath and was admitted for management of NSTEMI with a peak troponin I of 12.98 ng/mL. Other significant laboratory values included magnesium level of 1.2 mg/dL and potassium of 3.5 mEq/L. A few hours later, she went into ventricular fibrillation cardiac arrest presumed ischemic as well as in the setting of electrolyte abnormality. ROSC was achieved in 8 minutes. Electrocardiogram post-ROSC showed sinus tachycardia with non-specific lateral T waves changes. She emergently underwent coronary angiography which revealed critical ostial left main coronary artery (LMCA) 95% stenosis which was persistent despite nitroglycerin administration (Figure 3A). IVUS of the lesion revealed a minimal luminal area of atherosclerosis and extrinsic compression of LMCA between the pulmonary artery (PA) and coronary sinus (Figure 3B). The lesion was treated with Medtronic Resolute Onyx 3.5 mm × 18 mm DES with the restoration of TIMI III flow (Figure 4). Right heart catheterization (RHC) revealed a mean right atrial pressure of 12 mmHg, pulmonary artery pressure (PAP) of 61/32 mmHg with the mean of 45 mmHg, pulmonary capillary wedge pressure (PCWP) of 28 mmHg. Computed tomography angiography (CTA) later in the admission course revealed dilated PA most likely secondary to her severe pulmonary hypertension (Figure 5) with a patent LMCA stent (Figure 6). The hospital course was complicated by worsening septic shock and decompensated cirrhosis and she was ultimately transitioned to comfort care and passed.
Visual timelines for both case 1 and case 2 are demonstrated in Figures 7,8 per CARE reporting guidelines.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patients or the relatives after all possible attempts were made. Our institution does not require ethical approval for reporting individual cases or case series. Informed consent for patient information to be published in this article was not obtained because both patients in this case series passed away from their comorbidities and no identifying information is used in our report.
Discussion
The incidence of LMCA compression in pulmonary artery hypertension (PAH) patients was estimated to be up to 44% in older studies evaluating patients with atrial septal defect (ASD) and PAH (3). However, in more recent reports, it is estimated to be 4.5–20% (4). It was found to be up to 40% in coronary angiograms of patients with angina (5).
The mechanism of LMCA compression in the setting of PAH is primarily related to compression of LMCA between the dilated PA and the sinus of Valsalva as shown in Figure 5B. The compression can lead to ongoing myocardial ischemia with its consequences of chest pain, dyspnea, decreased exercise tolerance, palpitations, and in rare instances heart failure, cardiogenic shock, and cardiac arrest (6). While angina is a common manifestation in patients with PAH, coronary angiography is typically not performed unless they present with other symptoms of ischemia. Therefore, in these patients, CAD and LMCA compression can often go undiagnosed (7). Case 2 had no prior history of documented PAH and her initial presentation of LMCA compression was ischemia-driven malignant arrhythmia leading to cardiac arrest.
In stable clinical settings, patients with a history of PAH presenting with symptoms of ischemia, a non-invasive method of elucidating the degree of extrinsic compression can be done using multidetector computed tomography (MDCT) or cardiac magnetic resonance imaging (CMRI). These imaging modalities can help identify the size of the pulmonary dilation and its association with the LMCA, along with the LMCA takeoff angle. In addition, it can evaluate CAD, the underlying cause of PA dilation, and ventricular function (8,9). Patients with high clinical suspicion for ischemia or with suspicious non-invasive findings should undergo cardiac catheterization for definitive diagnosis and treatment and IVUS imaging is recommended if extrinsic compression is suspected (6). Furthermore, IVUS can guide with identifying appropriate stent size as well as length of deployment.
Multiple correlations with the presence of LMCA compression in PAH patients have been identified. On MDCT, LMCA takeoff angle is measured in reference to the sinus of Valsalva and an angle of <60 degrees has been associated with a higher risk of LMCA compression (8,10). Furthermore, it is more likely with a PA diameter >40 mm or a PA to ascending aorta diameter ratio of >1.2 (5,11). In one cohort of 26 patients, it was not present in patients with PA diameter <40 mm or PA to ascending aorta diameter ratio <1.2 (4). Moreover, it appears to be more common in younger patients with a higher incidence in women (11). Case 2 had a dilated PA most likely secondary to pulmonary hypertension as shown in the heart catheterizations values, however, interestingly the PA diameter was less than 40 mm (32.2 mm) and PA to ascending aorta diameter was 0.87 (32.2/37). Yet, she had LMCA compression with myocardial ischemia.
Regarding the treatment of LMCA compression in the setting of PAH, there are no guidelines available regarding the optimal therapy. Revascularization with percutaneous coronary intervention (PCI) and stenting or CABG has been reported, with several reports of successful PCI with stenting with favorable results (5,8,9,12). In one report, Galiè et al. describe the safety and the efficacy of PCI with stenting in 45 patients with LMCA compression in the setting of PAH with favorable outcomes at 36 months follow-up period. In the same report, the rate of restenosis was only 11%, and suggested mechanisms included mechanical recoil, continued recompression, and in-stent-neo intimal hyperplasia, and they were all treated successfully with repeat PCI and stenting (5). In the largest prospective series of PCI stenting for LMCA compression in patients with PAH, it was deemed to be safe with a cumulative survival of 69% at a follow-up period of 5 years which is the longest follow-up period in this patients’ population (13).
From the available literature, we have learned a few pitfalls regarding PCI stenting for LMCA compression in PAH. Precise stent positioning is difficult in comparison to atherosclerotic plaque. Additionally, avoiding short stents, and the use of DES is recommended to decrease the risk of restenosis (13). The choice of the stent is typically at the discretion of the operator; however, the durability of the stents, concomitant medication regimen, and patient’s comorbidities should be considered. Moreover, it is important to note that bare-metal stents (BMS) have greater durability of radial force against extrinsic compression than DES, especially in a large LMC diameter. Additionally, BMS is preferred in patients with a history or risk of bleeding given a shorter course of dual antiplatelet therapy. On the other hand, DES is better in term of lowering the rate of in-stent restenosis (5). Regardless of the choice of the stent, it is important to note that, LMCA ostium stents are at a higher risk of displacement into the aorta (14). Although PCI with stenting has favorable procedural tolerance and outcomes, the long-term outcomes of this strategy are still not well established.
Revascularization by CABG has also been reported; however, most patients with PAH are usually high surgical candidates with increased mortality (15,16). In instances where the PA dilation is secondary to congenital heart disease or cardiac shunts, surgical intervention is the appropriate intervention to prevent progressive dilation of the PA. Lastly, in patients with PA dilation secondary to PAH, they should also be started on appropriate pulmonary hypertension therapies to treat the underlying cause of the dilation and to prevent future worsening dilation (10).
Compared to PA dilation, coronary arteries compression secondary to chest tubes is extremely rare. To the best of our knowledge, there are only a few reports in the literature of chest tube compression of the coronaries leading to ischemia. Moreover, there is scarce data regarding the complications of chest tubes post-operatively in cardiac patients. Reported complications included pneumothorax, drains entrapped under sternal wires, lung injury, perforation of the diaphragm, intestinal perforation, rupture of the vein graft, and in extremely rare instances extrinsic compression of the epicardial coronaries or grafts (17). External compression of coronary arteries from chest tubes or drains causing myocardial ischemia can present post-operatively in cardiac patients with different clinical scenarios that include hemodynamic instability, cardiogenic shock, increase in cardiac markers, and electrocardiogram (EKG) changes (17-21).
The treatment usually involves removal or adjustment of the offending drain, DES or BMS can be used however is not necessary as removing or adjusting the drain often results in resolution of blood flow (18). In our patient, the myocardial ischemia caused by external compression of the chest tube on the SVG presented as an extreme clinical presentation of malignant arrhythmia that required emergent coronary angiogram for diagnosis and treatment by adjusting the drain and placing DES, patency of SVG was demonstrated three days later on the repeat coronary angiogram as shown in Figure 2D. Therefore, caution is needed to the details of the chest tube placement and its mechanical complications, and a high degree of suspicion for such a complication is needed when the patient exhibits unexcepted post-operatively cardiac ischemia. Coronary angiogram allows for diagnosis and treatment in a timely manner.
Conclusions
Extrinsic compression of the coronaries secondary to PA dilation or mechanical obstruction from nearby chest tubes or drain can cause ischemia and lead to catastrophic consequences. High clinical suspicion is warranted for proper diagnosis and treatment. In suspected LMCA compression from dilated PA in PAH patients, a non-invasive imaging modality with MDCT or CMRI is recommended, however in urgent settings or high suspicion for ischemia, coronary angiogram with IVUS should be done. Despite the lack of guidelines on the treatment, PCI with stenting has been shown to be effective and safe. In cardiac patients, if ischemia is noted intra-operatively or in the postoperative period, mechanical compression of the coronaries from nearby chest tubes or drain should be considered and diagnosis with coronary angiogram is recommended. Appropriate adjustment or removal of the chest tube must be performed.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-111/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-22-111/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-111/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patients or the relatives after all possible attempts were made. Our institution does not require ethical approval for reporting individual cases or case series. Informed consent for patient information to be published in this article was not obtained because both patients in this case series passed away from their comorbidities and no identifying information is used in our report.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Virani SS, Alonso A, Aparicio HJ, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021;143:e254-743. [Crossref] [PubMed]
- Mechanic OJ, Gavin M, Grossman SA, et al. Acute Myocardial Infarction (Nursing). In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
- Mitsudo K, Fujino T, Matsunaga K, et al. Coronary arteriographic findings in the patients with atrial septal defect and pulmonary hypertension (ASD + PH)--compression of left main coronary artery by pulmonary trunk. Kokyu To Junkan 1989;37:649-55. [PubMed]
- Mesquita SM, Castro CR, Ikari NM, et al. Likelihood of left main coronary artery compression based on pulmonary trunk diameter in patients with pulmonary hypertension. Am J Med 2004;116:369-74. [Crossref] [PubMed]
- Galiè N, Saia F, Palazzini M, et al. Left Main Coronary Artery Compression in Patients With Pulmonary Arterial Hypertension and Angina. J Am Coll Cardiol 2017;69:2808-17. [Crossref] [PubMed]
- de Jesus Perez VA, Haddad F, Vagelos RH, et al. Angina associated with left main coronary artery compression in pulmonary hypertension. J Heart Lung Transplant 2009;28:527-30. [Crossref] [PubMed]
- Lee MS, Oyama J, Bhatia R, et al. Left main coronary artery compression from pulmonary artery enlargement due to pulmonary hypertension: a contemporary review and argument for percutaneous revascularization. Catheter Cardiovasc Interv 2010;76:543-50. [Crossref] [PubMed]
- Akbal OY, Kaymaz C, Tanboga IH, et al. Extrinsic compression of left main coronary artery by aneurysmal pulmonary artery in severe pulmonary hypertension: its correlates, clinical impact, and management strategies. Eur Heart J Cardiovasc Imaging 2018;19:1302-8. [Crossref] [PubMed]
- Lindsey JB, Brilakis ES, Banerjee S. Acute coronary syndrome due to extrinsic compression of the left main coronary artery in a patient with severe pulmonary hypertension: successful treatment with percutaneous coronary intervention. Cardiovasc Revasc Med 2008;9:47-51. [Crossref] [PubMed]
- Batra K, Saboo SS, Kandathil A, et al. Extrinsic compression of coronary and pulmonary vasculature. Cardiovasc Diagn Ther 2021;11:1125-39. [Crossref] [PubMed]
- Kajita LJ, Martinez EE, Ambrose JA, et al. Extrinsic compression of the left main coronary artery by a dilated pulmonary artery: clinical, angiographic, and hemodynamic determinants. Catheter Cardiovasc Interv 2001;52:49-54. [Crossref] [PubMed]
- Ikegami R, Ozaki K, Ozawa T, et al. Percutaneous Coronary Intervention for a Patient with Left Main Coronary Compression Syndrome. Intern Med 2018;57:1421-4. [Crossref] [PubMed]
- Saia F, Dall'Ara G, Marzocchi A, et al. Left Main Coronary Artery Extrinsic Compression in Patients With Pulmonary Arterial Hypertension: Technical Insights and Long-Term Clinical Outcomes After Stenting. JACC Cardiovasc Interv 2019;12:319-21. [Crossref] [PubMed]
- Adigopula S, Nsair A. Left Main Coronary Artery Stent Migration. N Engl J Med 2015;373:1957. [Crossref] [PubMed]
- Fujiwara K, Naito Y, Higashiue S, et al. Left main coronary trunk compression by dilated main pulmonary artery in atrial septal defect. Report of three cases. J Thorac Cardiovasc Surg 1992;104:449-52. [Crossref] [PubMed]
- Rich S, McLaughlin VV, O'Neill W. Stenting to reverse left ventricular ischemia due to left main coronary artery compression in primary pulmonary hypertension. Chest 2001;120:1412-5. [Crossref] [PubMed]
- Svedjeholm R, Håkanson E. Postoperative myocardial ischemia caused by chest tube compression of vein graft. Ann Thorac Surg 1997;64:1806-8. [Crossref] [PubMed]
- Langer NB, Nazif TM, Powers ME, et al. Cardiogenic Shock From Coronary Compression: A Difficult Diagnosis But Easy Fix. Ann Thorac Surg 2016;101:e111-3. [Crossref] [PubMed]
- Heestermans TM, Dambrink JH, Sie HT. Immediate myocardial infarction due to compression of a vein graft. Ann Thorac Surg 2009;87:e15. [Crossref] [PubMed]
- Knipp S, Massoudy P, Piotrowski JA, et al. Pitfall in coronary artery bypass surgery: poor flow of left internal mammary artery to left anterior descending artery graft due to compression by a chest drain. Eur J Cardiothorac Surg 2002;22:438. [Crossref] [PubMed]
- Beiras-Fernandez A, Möhnle P, Kopf C, et al. An uncommon cause of myocardial ischemia after coronary artery bypass grafting: "the dangerous drainage". Heart Surg Forum 2011;14:E200-1. [Crossref] [PubMed]
Cite this article as: Patel H, Essa A, Sharkawi M, Hiner E. Myocardial ischemia in the setting of extrinsic coronary compression: two case reports. AME Case Rep 2024;8:11.