Successful rituximab therapy in adult-onset IgA vasculitis with diffuse alveolar hemorrhage and renal failure: a case report
Highlight box
Key findings
• We describe a rare case of a patient with adult-onset immunoglobulin A vasculitis (IgAV) found to have acute renal failure and diffuse alveolar hemorrhage (DAH).
What is known and what is new?
• DAH is a rare and life-threatening manifestation of adult-onset IgAV. Due to the rarity of DAH in adult-onset IgAV, literature to guide management of the condition is limited.
• This case describes the successful use of Rituximab with steroids to treat concomitant DAH and renal failure in a patient with adult-onset IgAV.
What is the implication, and what should change now?
• Although DAH occurs rarely in patients with adult-onset IgAV, prompt recognition is imperative to guide successful treatment. Further research is necessary to determine the optimal therapeutic approach.
Introduction
Immunoglobulin A vasculitis (IgAV) is a small vessel vasculitis mediated by immunoglobulin A (IgA) complex deposition in the small vessels of the skin, gastrointestinal tract, joints, and kidneys, classically presenting with palpable purpura, abdominal symptoms, joint pain, and kidney disease (1). As IgAV is more common in children, adult-onset disease is rare but is often associated with more severe features, including renal and pulmonary involvement (2,3). Renal manifestations are more prevalent in adults and typically result in poor kidney outcomes as older age of onset is an independent risk factor for severe kidney disease. Less commonly, pulmonary findings, such as interstitial fibrosis, usual interstitial pneumonia, or diffuse alveolar hemorrhage (DAH), can develop and often indicate higher morbidity and mortality. We report a case of a woman with previously diagnosed adult-onset IgAV who presented with acute renal failure and alveolar hemorrhage. We present this article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-43/rc).
Case presentation
A 52-year-old, Caucasian woman with a history of IgAV, hypothyroidism, obesity, and former tobacco use presented to the emergency department with one month of lower extremity swelling, joint pain, and shortness of breath. She was diagnosed with IgAV two years prior following skin biopsy with findings of IgA and complement deposition on immunofluorescence. Additional studies at the time of diagnosis included an elevated erythrocyte sedimentation rate (ESR) of 128 mm/h (ref. 0–30 mm/h), IgA of 1,006 mg/dL (ref. 70–400 mg/dL), and immunoglobulin G of 2,276 mg/dL (ref. 700–1,600 mg/dL), along with proteinuria (100 mg/dL) and hematuria on urinalysis. Further autoimmune work-up, including anti-neutrophil cytoplasmic antibodies (ANCAs), was negative. Initial outpatient treatment included prednisone and azathioprine, although she quickly discontinued these medications within one year of initial diagnosis due to loss of insurance.
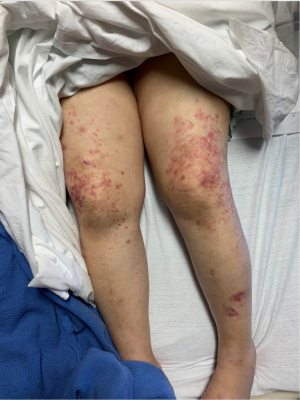
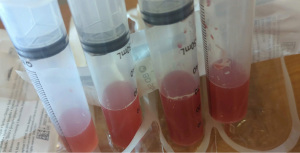
At the time of presentation, she reported one month of lower extremity pain and swelling, abdominal pain, bloody stools, new skin lesions, and progressive shortness of breath. She was febrile to 101.5 F, normotensive (blood pressure 110/70 mmHg), and tachycardic (heart rates 100–120 bpm). The physical exam was notable for diffuse abdominal tenderness and multiple purpuric lesions of varying size on the forearms and bilateral lower extremities (Figure 1). Initial labs were significant for macrocytic anemia with hemoglobin of 7.2 g/dL (ref. 11.2–15.7 g/dL) and mean corpuscular volume (MCV) of 120 fL (ref. 79.4–94.8 fL), thrombocytopenia with platelet count 134×103/µL [ref. (160–383)×103/µL], elevated NT-pro brain natriuretic peptide of 394 pg/mL (ref. 300 pg/mL), international normalized ratio of 2.1 (ref. 0.9–1.1), prothrombin time of 24 seconds (ref. 10.2–13 seconds), ESR of 122 mm/h (ref. 0–30 mm/h), C-reactive protein of 2.0 mg/dL (ref. <0.5 mg/dL), elevated immunoglobulin G of 2,040 mg/dL (ref. 700–1,600 mg/dL), normal complement component 3 of 94 mg/dL (ref. 90–180 mg/dL), and low complement component 4 of 9 mg/dL (ref. 10–40 mg/dL). The chest X-ray demonstrated hazy opacities in the bilateral lower lobes and hilar fullness concerning for mild pulmonary edema. Notable negative studies included antinuclear antibody (ANA), ANCA, anti-glomerular basement membrane (GBM) and cyclic citrullinated peptide (CCP) antibodies. In addition, microbiologic studies, thyroid function tests, hemolysis studies, and hepatitis serologies were unremarkable, along with a normal transthoracic echocardiogram without evidence of systolic and diastolic dysfunction or valvular abnormalities. She was given intravenous fluids and started on antibiotic therapy (vancomycin, cefepime, and metronidazole) for the treatment of presumed sepsis. On the second day of admission, laboratory testing revealed worsening anemia with a decrease in hemoglobin from 7.2 to 6.4 g/dL requiring transfusion of one unit of red blood cells (RBCs). Two days later the hemoglobin again dropped to 6.0 g/dL. In addition, she developed progressive renal failure with creatinine increasing from 0.99 mg/dL at presentation to 2.02 mg/dL associated with hematuria (78 RBCs per high power field), proteinuria (50 mg/dL, urine protein to creatinine ratio 0.30), and granular casts with dysmorphic RBCs seen on urine microscopy. That evening, she developed worsening oliguria, shortness of breath, and a new cough. Computed tomography (CT) of the chest, abdomen, and pelvis demonstrated bilateral, upper lung predominant confluent consolidative nodular opacities with sparing of the periphery (Figures 2,3), as well as bowel wall thickening of the proximal colon suggestive of colitis. Due to the development of acutely worsening respiratory failure, she was transferred to the medical intensive care unit, intubated, and started on continuous renal replacement therapy. Bronchoscopy with bronchoalveolar lavage (BAL) was performed and revealed progressively more hemorrhagic effluent with each aliquot (11,650, 17,600 and 25,200 RBC/mcL) as demonstrated in Figure 4.
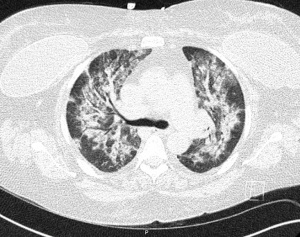
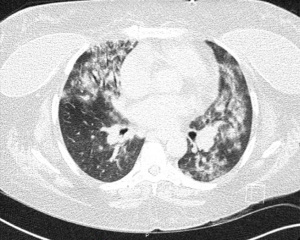
Given the constellation of symptoms and laboratory findings, including rapidly progressive respiratory failure, consolidative opacities seen on imaging, a drop in hemoglobin count, microhematuria, and creatinine elevation, her presentation was felt to be consistent with DAH and acute renal failure secondary to recrudescence of systemic IgAV. Due to the severity of her presentation and the rapid progression of symptoms, immediate treatment was warranted without confirmatory renal biopsy. She received 1 gram of rituximab (RTX) and methylprednisolone (250 mg every 6 hours for 5 days). One week later, she was extubated and transitioned to intermittent hemodialysis (iHD).
Notably, her hospital course was complicated by candidemia treated with a 14-day course of fluconazole, ultimately delaying the second dose of RTX to 4 weeks after the initial dose. Her steroid course, however, was continued without interruption. Following an initial pulse dose of methylprednisolone 250 mg every 6 hours for 5 days, the methylprednisolone dose was halved every 2 days (e.g., from 250 mg every 6 hours to 125 mg every 6 hours) until a dose of methylprednisolone 60 mg every 12 hours was reached. After 48 hours on this methylprednisolone dose, she was then transitioned to prednisone at a dose of 60 mg daily. Prednisone was gradually decreased every 2 weeks to 20 mg daily over a total period of 6 weeks.
With these interventions, she developed full respiratory and renal recovery [creatinine (Cr) peaked at 5.68 ng/dL between iHD sessions and returned to a baseline of 0.5–0.7 mg/dL when iHD was held 4 weeks after initiation of immunosuppression] and was transferred to inpatient rehabilitation due to critical illness myopathy and polyneuropathy. Following two weeks of intensive therapy, she had marked improvement in her functional status and was discharged home with her family on a maintenance dose of Prednisone 20 mg daily. Outpatient follow-up with rheumatology was scheduled for discussions regarding further treatment although the patient was lost to follow up.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Adult-onset IgAV is a rare disease with an annual incidence of approximately 5.1 per 100,000 adults (4). In addition to the typical dermatological, joint, and gastrointestinal symptoms, more severe features, such as renal and pulmonary involvement, can be seen in adults and are often associated with a poor prognosis.
Pulmonary involvement in adult-onset IgAV is a very infrequent phenomenon. A retrospective review of all adult patients seen at the Mayo Clinic over a 6-year period identified 124 total patients with IgAV, of which only 3 (2.4%) demonstrated pulmonary involvement (5). Pulmonary manifestations described in the literature include usual interstitial pneumonia, interstitial fibrosis, and DAH. In a systematic review of 36 patients with DAH in IgAV, patients were found to be older (median age 16.5 years) and often presented with a hemoglobin drop (74%), hemoptysis (75%), and chest infiltrates (94%). The presentation was often severe with approximately 50% of patients requiring mechanical ventilation, suggesting frequently delayed recognition of alveolar hemorrhage (6). The co-occurrence of renal manifestations, including nephrotic syndrome, diffuse proliferative glomerulonephritis, and acute renal failure, is common and often confers a poor prognosis (7).
Formal diagnosis of DAH requires bronchoscopy with serial BAL demonstrating either (4) progressively more hemorrhagic lavage fluid with each instillation and/or (5) hemosiderin laden macrophages on cytology (8). The phenotype of DAH encompasses a number of distinct immune and non-immune pathologic entities including pulmonary capillaritis, bland pulmonary hemorrhage, and diffuse alveolar damage (9). Of these etiologies, pulmonary capillaritis is the most common, responds to immunosuppression (10), and is generally associated with systemic vasculitides and connective tissue disorders (11).
The literature guiding the treatment of adult-onset IgAV is limited as data is often extrapolated from studies performed in pediatric populations or patients with isolated IgA nephropathy. Severe IgAV has historically been treated using a combination of corticosteroids and/or immunosuppressive agents, such as cyclophosphamide or RTX. Although cyclophosphamide is commonly used, a randomized study performed in adults with IgAV and renal involvement did not demonstrate improved renal outcomes or patient survival with cyclophosphamide therapy (12). In contrast, emerging evidence supports the use of RTX as a safe and effective therapeutic option for pediatric and adult patients with resistant or refractory IgAV (13-15). Due to the role of B lymphocytes in the pathogenesis of IgAV, RTX may reduce the formation of IgA containing immunocomplexes and limit IgAV disease activity. The dosing regimen for our patient was based upon a small series of adult IgAV patients successfully treated with glucocorticoids and RTX (16). Given the rarity of DAH in IgAV, consensus treatment guidelines do not exist, and management decisions are often extrapolated from the treatment of severe IgAV, as discussed previously, or presentations of DAH in ANCA-associated vasculitis which have recommended initiation of induction therapy with RTX in combination with pulse glucocorticoid therapy (17). Although plasma exchange (PLEX) is recommended as first-line therapy in some forms of vasculitis, such as anti-GBM disease and severe hepatitis B-related polyarteritis nodosa, there is no robust data to support the use of PLEX in IgAV (18).
Our case demonstrates the uncommon presentation of severe IgAV with DAH and the efficacy of the combination of RTX and steroids in the treatment of severe IgAV. The patient carried a pre-established diagnosis of IgAV and presented with common symptoms seen in poorly controlled IgAV, including cough, fever, joint pain, abdominal pain, palpable purpura, and dyspnea. She developed a rapidly escalating oxygen requirement, acute hemoglobin drop, and bilateral pulmonary infiltrates seen on imaging. Although she did not experience hemoptysis, BAL revealed progressively more hemorrhagic effluent concerning for DAH. Her acute renal failure was concomitant with her respiratory decline and evidence of dysmorphic RBCs seen on urine microscopy. Due to the severity of her presentation, she was rapidly initiated on RTX, methylprednisolone, and renal replacement therapy without a kidney biopsy. She demonstrated marked improvement in both respiratory and renal function. Notably, she experienced complete renal recovery with sustained return to normal glomerular filtration rate (GFR) and resolution of hematuria. Her supplemental oxygen needs improved significantly as well, though with the important caveat that this improvement is difficult to definitively attribute to immunosuppression of a true pulmonary capillaritis given the simultaneous initiation of hemodialysis and immunosuppression.
Conclusions
This case highlights the importance of considering DAH as a rare and life-threatening pulmonary manifestation of adult-onset IgAV. Although pulmonary renal syndromes are more commonly seen in other rheumatic diseases, such as ANCA-associated vasculitides, it is imperative to recognize the less common association between IgAV and alveolar hemorrhage, which is associated with high morbidity and mortality. In addition, this case demonstrates the use of RTX as an effective therapeutic option in a patient presenting with severe manifestations of IgAV.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-43/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-43/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-43/coif). S.K. serves as the chair of the American Board of Internal Medicine Rheumatology Board which provides an honorarium as well as reimbursement for travel and lodging, and reports grant support from The Arthritis Foundation which ended in December 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Audemard-Verger A, Pillebout E, Guillevin L, et al. IgA vasculitis (Henoch-Shönlein purpura) in adults: Diagnostic and therapeutic aspects. Autoimmun Rev 2015;14:579-85. [Crossref] [PubMed]
- Batu ED, Sarı A, Erden A, et al. Comparing immunoglobulin A vasculitis (Henoch-Schönlein purpura) in children and adults: a single-centre study from Turkey. Scand J Rheumatol 2018;47:481-6. [Crossref] [PubMed]
- Kang Y, Park JS, Ha YJ, et al. Differences in clinical manifestations and outcomes between adult and child patients with Henoch-Schönlein purpura. J Korean Med Sci 2014;29:198-203. [Crossref] [PubMed]
- Hočevar A, Rotar Z, Ostrovršnik J, et al. Incidence of IgA vasculitis in the adult Slovenian population. Br J Dermatol 2014;171:524-7. [Crossref] [PubMed]
- Nadrous HF, Yu AC, Specks U, et al. Pulmonary involvement in Henoch-Schönlein purpura. Mayo Clin Proc 2004;79:1151-7. [PubMed]
- Rajagopala S, Shobha V, Devaraj U, et al. Pulmonary hemorrhage in Henoch-Schönlein purpura: case report and systematic review of the english literature. Semin Arthritis Rheum 2013;42:391-400. [Crossref] [PubMed]
- Rajagopala S, Parameswaran S, Ajmera JS, et al. Diffuse alveolar hemorrhage in IgA nephropathy: case series and systematic review of the literature. Int J Rheum Dis 2017;20:109-21. [Crossref] [PubMed]
- Maldonado F, Parambil JG, Yi ES, et al. Haemosiderin-laden macrophages in the bronchoalveolar lavage fluid of patients with diffuse alveolar damage. Eur Respir J 2009;33:1361-6. [Crossref] [PubMed]
- Samuel S, Brown B, Mason N, et al. Diffuse alveolar hemorrhage, a rare presentation of polymyositis. Respir Med Case Rep 2020;31:101261. [Crossref] [PubMed]
- Travis WD, Colby TV, Lombard C, et al. A clinicopathologic study of 34 cases of diffuse pulmonary hemorrhage with lung biopsy confirmation. Am J Surg Pathol 1990;14:1112-25. [Crossref] [PubMed]
- de Prost N, Parrot A, Picard C, et al. Diffuse alveolar haemorrhage: factors associated with in-hospital and long-term mortality. Eur Respir J 2010;35:1303-11. [Crossref] [PubMed]
- Pillebout E, Alberti C, Guillevin L, et al. Addition of cyclophosphamide to steroids provides no benefit compared with steroids alone in treating adult patients with severe Henoch Schönlein Purpura. Kidney Int 2010;78:495-502. [Crossref] [PubMed]
- Maritati F, Fenoglio R, Pillebout E, et al. Brief Report: Rituximab for the Treatment of Adult-Onset IgA Vasculitis (Henoch-Schönlein). Arthritis Rheumatol 2018;70:109-14. [Crossref] [PubMed]
- Maritati F, Canzian A, Fenaroli P, et al. Adult-onset IgA vasculitis (Henoch-Schönlein): Update on therapy. Presse Med 2020;49:104035. [Crossref] [PubMed]
- Hernández-Rodríguez J, Carbonell C, Mirón-Canelo JA, et al. Rituximab treatment for IgA vasculitis: A systematic review. Autoimmun Rev 2020;19:102490. [Crossref] [PubMed]
- Fenoglio R, Naretto C, Basolo B, et al. Rituximab therapy for IgA-vasculitis with nephritis: a case series and review of the literature. Immunol Res 2017;65:186-92. [Crossref] [PubMed]
- Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010;363:221-32. [Crossref] [PubMed]
- Régent A, Mouthon L, Guillevin L, et al. Role of therapeutic plasma exchanges in systemic vasculitis. Transfus Apher Sci 2020;59:102992. [Crossref] [PubMed]
Cite this article as: Ghebranious M, Leslie M, Manthuruthil C, Dhamankar O, Kazi S. Successful rituximab therapy in adult-onset IgA vasculitis with diffuse alveolar hemorrhage and renal failure: a case report. AME Case Rep 2024;8:12.