
Spinal subarachnoid hemorrhage progressing to spinal cord compression in a patient on a direct oral anticoagulant: a case report
Highlight box
Key findings
• A rare case of spontaneous spinal subarachnoid hemorrhage (SSH) occurred in an elderly woman on anticoagulant therapy, marked by sudden chest and back pain escalating to neurological deficits.
What is known and what is new?
• SSH is linked with high morbidity and mortality.
• This report underscores the heightened risk of SSH in anticoagulated elderly patients presenting with sudden back pain and neurological dysfunction.
What is the implication, and what should change now?
• Medical personnel should remain alert for SSH signs in anticoagulated patients to facilitate early diagnosis and intervention.
• This case advocates for prompt action to avert permanent neurological damages.
• A call for increased awareness and research is made to enhance understanding and improve SSH patient outcomes.
Introduction
Spinal subarachnoid hemorrhage (SSH) is a rare but known entity that can cause severe and irreversible motor, sensory and autonomic dysfunction if not decompressed in a timely manner. SSH occurs in <1% of all patients with subarachnoid hemorrhage (SAH) (1).
Tumors, trauma, arteriovenous malformations (AVM) and aneurysms may all cause spinal hemorrhage. Other entities that have been associated with spinal hemorrhage are blood dyscrasias, anticoagulation therapy, vasculitis and coarctation of the aorta (1-4). SSH is the least common form of spinal hemorrhage and consists 15% of all spinal hematomas (3). When SSH does occur, it is most frequently observed in the thoracic segment of the subarachnoid space (5). In rare cases, SSH can spread into the intracranial subarachnoid space because of the anatomical continuity between them (6).
SSH leading to spinal cord compression is a rare occurrence, but healthcare providers should remain vigilant due to its potential implications. Prompt intervention and favorable preoperative clinical status play a pivotal role in achieving successful recovery (7). Existing literature primarily consists of isolated case reports, with many cases being diagnosed only after the onset of symptoms. Consequently, the epidemiology, optimal management strategies and outcomes remain largely unexplored. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-68/rc).
Case presentation
An 82-year-old woman presented to the emergency department with radiating chest and epigastric pain, extending to the upper and lower back, after providing testimony in court earlier that day. The patient had been taking 5 mg apixaban twice daily due to a diagnosis of atrial fibrillation. She also had a medical history of ischemic heart disease, hypertension and osteoporosis, and had received a cardiac pacemaker eight months prior due to an atrioventricular block. Upon intake, physical examination and initial cardiac workup were remarkable only for elevated systolic blood pressure (189/72 mmHg) and respiratory alkalosis.
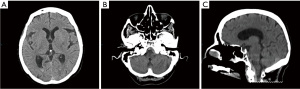
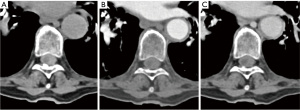
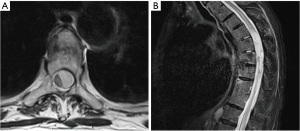
During a reevaluation in the emergency room a few hours later, the patient exhibited new bilateral lower extremity weakness, sensory changes, urinary and fecal incontinence, and a positive Babinski reflex. A non-contrast head computed tomography (CT) revealed fluid-blood levels in the occipital horns of the lateral ventricles (Figure 1A), blood in the subarachnoid space of the left posterior fossa and peripheral hyper-density at the craniocervical transition (Figure 1B,1C), suggestive of peri-mesencephalic SAH. Due to worsening neurological symptoms and chest pain, a CT angiography (CTA) of the neck, chest and abdomen was done, to rule out an aortic dissection. No vascular abnormalities were observed on concurrent CTA, however, SSH was demonstrated throughout the spine, most prominent on the non-contrast scans as a hyperdensity surrounding the spinal cord (Figure 2). Magnetic resonance imaging (MRI) scan of the thoracic spine was further done after having the patient’s pacemaker programmed appropriately, to assess the extent of hemorrhage and to look for potential etiology. The MRI confirmed SSH hemorrhage with significant cord compression in the thoracic regions T6–T9 (Figure 3). The hemorrhage extended from the cranio-cervical junction to the terminus of the spinal canal and was also evident circumferentially surrounding the entire length of the spinal cord. Notably, there was an area of asymmetrical compression between T6–T9 vertebrae, likely due to a blood clot that had developed in that region, causing significant cord compression in the thoracic region. The intracranial subarachnoid hemorrhagic component further supported the diagnosis. On T2-weighted images, subtle cord signal changes suggest mild myelopathic changes. No etiological factor was identified in neither of the scans.
Preoperatively, anticoagulant reversal was sought with Prothrombin complex concentrate, and physical examination showed flaccid paraparesis in both legs with a power grading of 0/5 in the L2–L4 myotomes bilaterally, and 1–2/5 power in the L5–S1 myotomes bilaterally. The patient underwent an urgent thoracic laminectomy, which confirmed the presence of hemorrhage in the subarachnoid space. A dorsally located subarachnoid hematoma at T6–T9 level was evacuated. Post-surgery, the patient experienced partial restoration of strength; lower right limb power improved to 4/5 proximally, and 5/5 distally, and 4/5 in the distal left limb, but residual weakness persisted in the proximal left lower limb (1/5). During the post-operative hospitalization, persistent headache and nuchal rigidity prompted an additional CT scan, which showed improvement compared to previous imaging. Subsequently, a lumbar puncture was performed, and antibiotics were initiated. The analysis of cerebrospinal fluid (CSF) was normal, leading to the discontinuation of antibiotics and the diagnosis of chemical meningitis. The patient was then transferred to an in-hospital rehabilitation setting, where she received low molecular weight heparin (LMWH) and underwent a tapering course of steroids. Due to an overall postsurgical improvement post and no vascular abnormality found on imaging or intraoperatively, angiography was not completed.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made, due to loss to follow-up.
Discussion
SSH is a rare clinical condition whose etiology is not entirely clear. It is believed that minor trauma can lead to increased pressure within the spinal vessels, particularly the valveless radiculomedullary veins located in the subarachnoid space (8). This elevated pressure can cause vessel rupture (8). On the other hand, hemorrhage may also originate from the subdural space where blood can pass through the arachnoid membrane, resulting in an associated SAH. SSH rarely presents as a hematoma owing to the diluting and redistributing effect of the CSF, unless the hematoma is sufficiently large to block the CSF flow (9).
Anticoagulant therapy is a complex endeavor that often faces challenges due to the occurrence of hemorrhage. The delicate balance between the appropriate use of anticoagulants and the potential life-threatening consequences of their insufficient or excessive administration must be carefully managed. The risks associated with thromboembolism and bleeding heavily rely on the specific anticoagulation treatment employed. Hemorrhages induced by anticoagulants can affect the craniospinal axis, even in the absence of any traumatic events, and are associated with medications such as warfarin, heparin and direct oral anticoagulants (DOACs) (10).
While anticoagulant-associated intracranial hemorrhage typically manifests as intracerebral bleeding (11), the spinal presentation commonly presents as epidural bleeding (3), which can rapidly progress and necessitate immediate emergency surgical intervention. Literature has documented the occurrence of spontaneous spinal epidural hematomas associated with DOAC (12-14). However, we only found one additional reported case of a spontaneous SSH secondary to a DOAC, specifically apixaban (15). This unique case involved a patient who was concurrently taking apixaban and clopidogrel, with imaging revealing cortical superficial siderosis. Therefore, the case we have reported represents the first documented instance of spontaneous SSH attributed to apixaban, adding to the existing body of knowledge on the topic.
Differentiating between epidural, subdural, and SSHs can be a challenge, solely based on clinical manifestations. All three forms may present with severe back pain, frequently referred to the level of the lesion, may radiate to both legs, flanks, chest or abdomen, and may be concurrent with neurological deficits (3). As such, the possibility of SSH must be contemplated in patients exhibiting new-onset limb weakness or sensory impairment coupled with pain. Indicators of SAH typically encompass meningism, headaches, and alterations in mental status (9,16). However, clinical presentation and prognosis can vary depending on the hemorrhage’s position within the spinal canal. Thecal sac compression is usually resultant from dorsally located SSH, whereas ventral hemorrhages are generally not associated with neurological decline (1).
The diagnosis of SSH continues to be difficult despite advancements in neuroimaging. A lumbar puncture can assist in SSH diagnosis, but potential misinterpretation as a puncture injury may cause delays. Spinal angiography is generally considered to be the gold standard for demonstrating spinal artery aneurysms, AVM or another pathology that can cause SSH (17). MRI is excellent for determining hemorrhage extent and its relationship with the thecal sac, monitoring treatment, and depicting the temporal changes of hemorrhage (18).
Prompt identification of spinal hemorrhage is important for the emergent treatment of patients and the preservation of normal function. One study reported that most cases were diagnosed based on surgical or autopsy findings (2). Therefore, SSH should not be missed in patients with acute paraplegia, especially in those with accompanying back pain or headache.
The treatment for SSH depends on the presence or absence of neurological deficits. Surgery is indicated in the presence of a mass effect with spinal cord compression, severe neurological dysfunction or deteriorating neurological condition (2,19). In the absence of severe neurological symptoms, SSH can be managed conservatively (1). Because our patient experienced acute neurological deterioration surgical intervention was deemed necessary. Prognosis after surgical intervention varies and depends on the severity of neurological impairment, the duration between the onset of symptom and surgical intervention and the rate of neurological deterioration (7). When surgery is indicated, surgical intervention within twelve hours of symptomatic onset has more than twice the recovery rate, in comparison to patients that received treatment within thirteen hours to one week (3).
After a SAH, there is an increase in reactive oxygen species (ROS) production, which can cause lipid peroxidation and DNA/protein breakdown. This damage, combined with the roles of nitric oxide (NO) and endothelin-1 (ET-1), contributes to cerebral vasospasms (20). Although the degree of vasospasm in patients specifically suffering from a spinal SAH remains elusive, the prevailing management strategies involve the use of Nimodipine. This calcium channel antagonist is frequently prescribed due to its neuroprotective properties. Other drugs, like dexmedetomidine, selective serotonin reuptake inhibitors (SSRIs), and DL-3-n-butylphthalide, have shown promise in enhancing lymphatic flow and aiding the recovery process (21).
Conclusions
Anticoagulant-induced spinal hemorrhage should be included in the differential diagnosis for patients presenting with signs of acute spinal cord compression. MRI is the imaging modality of choice and should be performed without delay to confirm the diagnosis. Findings on a CT scan obtained as part of the workup can support the diagnosis of SSH and expedite further imaging studies and relevant consults. If there is no early spontaneous recovery, emergency surgical evacuation of the hematoma offers the best chance of recovery. Patients on DOACs require the additional consideration of optimal timing, risk assessment of intra-operative bleeding, understanding of the pharmacokinetics of the DOAC used and possible reversal options available.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-68/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-68/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-68/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made, due to loss to follow-up.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Komiyama M, Yasui T, Sumimoto T, et al. Spontaneous spinal subarachnoid hematoma of unknown pathogenesis: case reports. Neurosurgery 1997;41:691-3; discussion 693-4. [PubMed]
- Cihangiroglu M, Bulut S, Nayak S. Spinal subarachnoid hemorrhage complicating oral anticoagulant therapy. Eur J Radiol 2001;39:176-9. [Crossref] [PubMed]
- Kreppel D, Antoniadis G, Seeling W. Spinal hematoma: a literature survey with meta-analysis of 613 patients. Neurosurg Rev 2003;26:1-49. [Crossref] [PubMed]
- Domenicucci M, Ramieri A, Ciappetta P, et al. Nontraumatic acute spinal subdural hematoma: report of five cases and review of the literature. J Neurosurg 1999;91:65-73. [PubMed]
- Pullarkat VA, Kalapura T, Pincus M, et al. Intraspinal hemorrhage complicating oral anticoagulant therapy: an unusual case of cervical hematomyelia and a review of the literature. Arch Intern Med 2000;160:237-40. [Crossref] [PubMed]
- Nam KH, Lee JI, Choi BK, et al. Intracranial extension of spinal subarachnoid hematoma causing severe cerebral vasospasm. J Korean Neurosurg Soc 2014;56:527-30. [Crossref] [PubMed]
- Sunada I, Akano Y, Kidosaki Y, et al. Spontaneous spinal subarachnoid hematoma--case report. Surg Neurol 1995;44:133-6. [Crossref] [PubMed]
- Morandi X, Riffaud L, Chabert E, et al. Acute nontraumatic spinal subdural hematomas in three patients. Spine (Phila Pa 1976) 2001;26:E547-51. [Crossref] [PubMed]
- Kyriakides AE, Lalam RK, El Masry WS. Acute spontaneous spinal subdural hematoma presenting as paraplegia: a rare case. Spine (Phila Pa 1976) 2007;32:E619-22. [Crossref] [PubMed]
- Tomaselli GF, Mahaffey KW, Cuker A, et al. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2020;76:594-622. [Crossref] [PubMed]
- Morotti A, Goldstein JN. Anticoagulant-associated intracerebral hemorrhage. Brain Hemorrhages 2020;1:89-94. [Crossref]
- Ismail R, Zaghrini E, Hitti E. Spontaneous Spinal Epidural Hematoma in a Patient on Rivaroxaban: Case Report and Literature Review. J Emerg Med 2017;53:536-9. [Crossref] [PubMed]
- Bamps S, Decramer T, Vandenbussche N, et al. Dabigatran-associated spontaneous acute cervical epidural hematoma. World Neurosurg 2015;83:257-8. [Crossref] [PubMed]
- Goldfine C, Glazer C, Ratzan RM. Spontaneous Spinal Epidural Hematoma from Rivaroxaban. Clin Pract Cases Emerg Med 2018;2:151-4. [Crossref] [PubMed]
- Heckmann JG. Spinal subarachnoid hemorrhage in cortical superficial siderosis after apixaban and clopidogrel therapy. J Thromb Thrombolysis 2016;41:654-5. [Crossref] [PubMed]
- Gaitzsch J, Berney J. Spinal subarachnoid hematoma of spontaneous origin and complicating anticoagulation. Report of four cases and review of the literature. Surg Neurol 1984;21:534-8. [Crossref] [PubMed]
- Berlis A, Scheufler KM, Schmahl C, et al. Solitary spinal artery aneurysms as a rare source of spinal subarachnoid hemorrhage: potential etiology and treatment strategy. AJNR Am J Neuroradiol 2005;26:405-10. [PubMed]
- Kim YH, Cho KT, Chung CK, et al. Idiopathic spontaneous spinal subarachnoid hemorrhage. Spinal Cord 2004;42:545-7. [Crossref] [PubMed]
- Ichiba T, Hara M, Nishikawa K, et al. Comprehensive Evaluation of Diagnostic and Treatment Strategies for Idiopathic Spinal Subarachnoid Hemorrhage. J Stroke Cerebrovasc Dis 2017;26:2840-8. [Crossref] [PubMed]
- Tabarestani A, Patel A, Reddy A, et al. Vasospasm Management Strategies. Int J Med Pharm Res 2023;4:150-60. [PubMed]
- Hosseini Siyanaki MR, Lucke-Wold B, Khan M. Exploration of treatments for subarachnoid hemorrhage. J Biomed Res (Middlet) 2022;3:48-55. [PubMed]
Cite this article as: Wexler A, Hijazi M, Neeman B. Spinal subarachnoid hemorrhage progressing to spinal cord compression in a patient on a direct oral anticoagulant: a case report. AME Case Rep 2024;8:2.


