
Right upper lobectomy after immunotherapy for primary malignant melanoma of the lung: a case report and literature review
Highlight box
Key findings
• Right upper lobectomy after immunotherapy for primary malignant melanoma of the lung (PMML).
What is known and what is new?
• Compared to cutaneous melanoma, mucosal melanoma has a different biology and clinical appearance. And there are no standards for the diagnosis and treatment of PMML. But nivolumab may be effective in mucosal melanoma regardless of the tumor molecular profile, similar to the demonstrated efficacy of nivolumab in cutaneous melanoma regardless of BRAF mutation status.
• We present the first instance of surgically treated PMML following programmed cell death 1 treatment.
What is the implication, and what should change now?
• The preoperative immune system in combination with a surgical procedure may boost patients’ chances of survival.
Introduction
The disease of malignant melanoma is challenging to treat. It typically manifests as a primary skin tumor, but it can also affect other organs and tissues, such as the oral cavity, paranasal sinuses, esophagus, larynx, vagina, and anorectum (1). While most involvement of the respiratory system is metastatic, primary malignant melanoma of the lung (PMML) is extremely uncommon, making up about 0.01% of all primary lung malignancies (2). Less than 80 occurrences have thus far been documented in English-language literature. The majority of these cases are PMML case reports (3). In this article, we describe a female patient with PMML. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-79/rc).
Case presentation
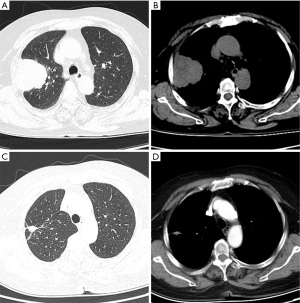
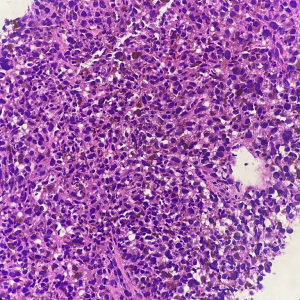
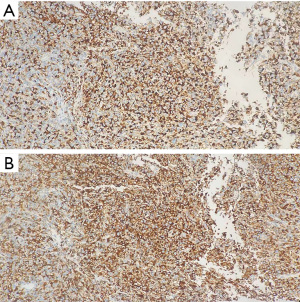
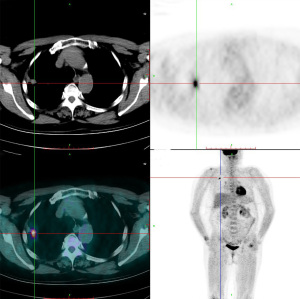
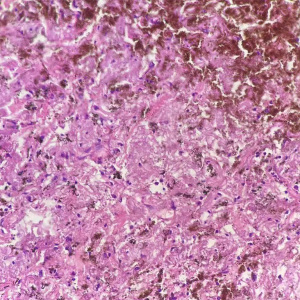
The 62-year-old female patient presented to Northern Jiangsu People’s Hospital (Yangzhou, China) with the right upper lung lobe occupancy found on health physical examination. She had no notable past medical history and had never smoked. She denied having ever had skin, mucous membranes, or eye surgery, or having any family history of the disease. Upon physical examination, both lungs were found to have clean breath sounds, and other body parts, such as skin, head, neck, scalp, anogenital area, and eyes, all appeared to be normal, thus excluding skin, uveal, or other mucosal lesions. A right upper lobe shadow with lobulated soft tissue density that measured roughly 54 mm × 50 mm was visible on a computed tomography (CT) plain and contrast-enhanced scan (such as Figure 1). According to laboratory testing, neuron-specific enolase (NSE) was 31.5 µg/L, which was greater than the standard value of 17 µg/L. Antibodies to tuberculosis were negative, and normal levels of carbohydrate antigen 125 (CA125), alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), cytokeratin fragment antigen 21-1 (CYFRA21-1), and progastrin-releasing peptide (proGRP) were seen. The following procedure was a CT-guided percutaneous lung biopsy. Hematoxylin and eosin (HE) staining revealed It consists of loosely adherent epithelioid cells. Some of the cells were seen to have large and round nuclei with deep staining, nuclear deviation, nuclear division, markedly eosinophilic nuclei, and eosinophilic cytoplasm. A large number of melanin granules were seen in the cytoplasm of the tumor cells. (Figure 2). Immunohistochemistry showed positive for human melanoma black 45 (HMB-45) and Melan-A (Figure 3), weakly positive for S-100 while pan-cytokeratin (CKpan), napsin, transcription termination factor 1 (TTF-1), CD45, p40, and p63 were all negative. Based on the clinical presentation, CT, other symptoms, and pathological analysis, the final diagnosis of PMML was made. A whole-body bone scan revealed no signs of potential bone metastases. No particular abnormalities were seen, according to a magnetic resonance imaging (MRI) of the brain and a CT scan of the abdomen. Plain CT revealed significant contact with the expectedly adherent chest wall, and enhanced CT revealed no discernible rib involvement, hence we advised surgical intervention for the patient. However, after knowing that surgery could result in a wide range of chest wall resections, varying degrees of chest wall adhesion, was complicated, and had a higher likelihood of requiring a thoracotomy, the patient made the decision to postpone surgery. Since B-Raf proto-oncogene (BRAF) mutation and other similar genetic tests take longer to produce findings, we decided to postpone mutation-related gene testing for the time being. A study has shown that Nivolumab may be effective in mucosal melanoma regardless of the tumor molecular profile, similar to the demonstrated efficacy of nivolumab in cutaneous melanoma regardless of BRAF mutation status (4). We suggested programmed cell death 1 (PD-1) treatment for her in light of the available research on cutaneous melanoma. However, nivolumab is pricey. And in 2020, In our hospital, there is just one PD-1, named sindilizumab (200 mg/3 weeks intravenous drip). She asked for therapy with sindilizumab after knowing about the cost and the drug’s uses. Yervoy was not yet listed in China at that time, hence it is not now regarded as a combo drug. The tumor dramatically decreased after four rounds of therapy; a CT scan revealed a 28 mm × 19 mm tumor. Twenty-nine cycles of treatment followed, and the rate of tumor shrinkage slowed down. Finally, a CT scan was done after 33 cycles of therapy and a total of 26 months, and it revealed a lump measuring roughly 16 mm × 10 mm in the right upper lung lobe (Figure 1). Whole-body positron emission tomography (PET)/CT was performed prior to surgery to determine the risk of the procedure and to detect a malignant occupying lesion in the right upper lung without detecting a potential original tumor elsewhere (Figure 4). Tumor volume gradually shrunk in patients receiving immunological treatment. As the patient’s confidence in their capacity to recover rose, so did their desire for surgery. Therefore, we performed a right upper lung lobectomy with lymph node dissection (stations 2R, 4R, and 7, 9, 10, 11 as well as parabronchial lymph nodes) in the patient. We found during the operation that the chest wall and the lung tissue where the tumor was located were closely connected. We removed and biopsied the surrounding chest wall tissue in order to avoid residual melanoma tissue, however, no cancer cells were discovered in the chest wall tissue (Figure 5). Loosely adherent epithelioid cells were dispersed across the target area after surgery, according to post-operative histological HE staining. Melanin is deposited in great quantities. Production of necrosis in the focus region. Inflammatory cells including lymphocytes and plasma cells have entered the surrounding fibrous tissue, causing it to become hyperplastic, and melanosis was seen in group 10 lymph nodes (Figure 6). Following surgery, immunotherapy was continued for adjuvant therapy. During the 6 months of post-operative follow-up, CA125, AFP, CEA, NSE, CYFRA21-1, proGRP, and thymidine kinase 1 (TK1) were within the normal range. The patient was disease-free and in great clinical condition.
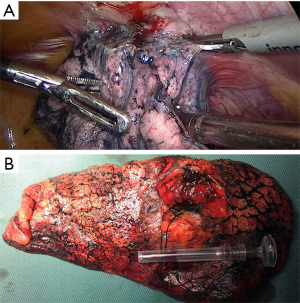
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Even though PMML is extremely uncommon, it is distinguished by high malignancy, a high risk of recurrence, and a dismal prognosis. In a review, 76 PMML patients with a median age of 60 [interquartile range (IQR), 51.25–68] years old, 61.84% of them were men, were listed (3). The clinical signs and symptoms of PMML, which typically affect the bronchi, are comparable to those of other thoracic malignancies, including cough, hemoptysis, lobe atrophy, dyspnea, obstructive pneumonia, and chest pain. Filippini et al. reported an interesting case of black sputum, which certainly represents a characteristic but rare clinical manifestation of PMML (5).
As we learn more about PMML, the diagnostic standards are evolving. It is challenging to distinguish between primary melanoma at non-cutaneous sites and metastatic lesions because cutaneous melanoma can spontaneously regress after metastasis, hiding the real primary site of the tumor (6). To exclude lung metastases, it is critical to get an accurate history of any prior melanotic skin lesions and to conduct a complete physical examination. Therefore, several authors have recommended specific criteria to diagnose pulmonary primary melanoma, and Jensen and Egedorf (7) suggested six criteria to specifically diagnose PMML: (I) no previously removed skin tumors; (II) no previously removed ocular tumors; (III) a solitary lung tumor; (IV) tumor morphology compatible with a primary tumor; (V) no other organ involvement; and (VI) pathologically, a diagnosis of malignant melanoma is made without primary malignant melanoma. Malignant melanoma is diagnosed pathologically when melanoma cells invade the bronchial epithelium, junctional changes occur, such as “dropping off” or “nesting” just below the epithelium, and tumor cells express HMB-45, S-100, Melan-A, and wave proteins, but not cytokeratin (CK), epithelial membrane antigen (EMA), chromogranin A (CgA), synaptophysin (Syn), human chorionic gonadotropin (hCG), high-molecular-weight-cytokeratin (HMW-CK), desmin, smooth muscle (SM)-actin, TTF-1, and small cell lung cancer (SCLC) (8). In our case, the positive staining for HMB-45 and Melan-A and weakly positive for S-100 contributed to the diagnosis of the disease. Immunohistochemical labeling, however, was not very effective in separating PMML from malignant melanoma at other sites. As a result, we had to show that there were no original lesions at cutaneous or other extracutaneous locations that were not pulmonary A comprehensive examination of the skin and eyes revealed no melanocytic lesions, and the results of the PET/CT scan revealed no indication of malignancy other than pulmonary malignancy. The patient in our case satisfied all requirements for a PMML diagnosis.
It is still debatable how PMML develops. Melanocytes are not present in the normal bronchial epithelium. However, there are several commonly accepted theories that seek to explain how primary malignant melanoma can arise in the lung. One hypothesis suggests that tumors arise from melanocytes that have migrated along with the primordial tubular respiratory tract during embryogenesis and are also present in the esophagus and pharynx (1,7). Another hypothesis suggests that epithelial cells undergo metaplastic transformation into melanocytes because squamous metaplasia is occasionally observed in the melanoma-affected epithelium (9). Additionally, it has also been proposed that neuroendocrine (Kultschitzky) precursor cells have the potential to undergo melanocytic differentiation. Both cell types are histogenetically related and of neural crest origin (10).
Since PMML does not have any precise guidelines. It is still unknown what the best course of action is for people with PMML. As a result, PMML uses the recommendations for cutaneous melanoma. Aggressive surgical procedures combined with radiation, chemotherapy, and immunotherapy are the available treatments for PMML. The primary form of treatment is surgery. Although some surgically treated patients pass away from metastasis and recurrence within a year of surgery, evidence shows that only individuals with surgically removed PMML have a possibility of long-term survival (11). Several studies have shown that lymph node involvement does not compromise long-term survival, and surgical resection may also be beneficial if the tumor subsequently proves to be an isolated metastasis from an occult primary melanoma (12). Radiotherapy at specific sites (head and neck) may be beneficial as palliative care to prevent or delay bleeding complications and neurological symptoms, which may reduce local recurrence rates but not prolong survival (13). The use of various chemotherapeutic agents, including dacarbazine and treatment with interleukin-2 (IL-2) or interferon (IFN), may occasionally have remarkable success in the treatment of cutaneous malignant melanoma, but the results are rather limited in the treatment of metastatic melanoma (14).
Notably, malignant melanoma is one of the fastest-responding cancer types to targeted therapy. Since the discovery of BRAF mutations in almost half of the melanoma patients, the United States (U.S.) Food and Drug Administration (FDA) has approved a series of BRAF inhibitors, including vemurafenib and dabrafenib, as well as mitogen-activated protein kinase kinase (MEK) inhibitors (15-17). With checkpoint inhibitors like ipilimumab [anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4)] and nivolumab (anti-PD-1), immunotherapy seems to be a promising option for the treatment of melanoma in numerous trials. The concurrent use of ipilimumab and nivolumab has manageable safety profiles and produced clinical data different from published monotherapy activity, achieving rapid and profound tumor regression (18). A study has also demonstrated the efficacy of post-operative surgery and the ability of anti-PD1 (pembrolizumab) to shrink mucosal melanoma to a size that allows for resection (19). The size of PMML decreased from 54 mm × 50 mm to 16 mm × 10 mm, a size that could be surgically removed. This proved that PD-1 works well for treating primary lung melanoma. The PD-1 pathway’s function in primary pulmonary melanoma, however, needs more research.
Melanoma is a tumor that cannot be considered cured even after a long disease-free period, and it can exhibit very delayed recurrence even after 40 years; therefore, it is important to regularly monitor these patients’ clinical symptoms, laboratory results, imaging studies, and other pertinent tests in order to identify metastatic spread and improve outcome. Shikuma et al. discovered that levels of 5-S-cysteine dopa (5-S-CD) might be used to track the effectiveness of treatment for primary pulmonary melanoma (20).
Conclusions
In our case report, we present the first instance of surgically treated PMML following PD-1 treatment. In addition, PD-1 immunotherapy has been shown to be successful in treating PMML. However, in order to substantiate only a few case reports, there must be several clinical trials. In our opinion, surgery is still the best option for treating resectable PMML. Patients who have tumors that are too large, difficult to remove cleanly, and who lack a strong desire for surgery may benefit first from immunotherapy for PD-1.
There is no question that the majority of the therapeutic methods currently available for PMML are derived from cutaneous melanoma therapies, even though the outcomes vary from treatment to treatment. Our case report enables physicians to provide a new therapeutic idea for the treatment of rare PMML and to properly balance the risks.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-79/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-79/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-79/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1998;83:1664-78. [Crossref] [PubMed]
- Wilson RW, Moran CA. Primary melanoma of the lung: a clinicopathologic and immunohistochemical study of eight cases. Am J Surg Pathol 1997;21:1196-202. [Crossref] [PubMed]
- Paliogiannis P, Fara AM, Pintus G, et al. Primary Melanoma of the Lung: A Systematic Review. Medicina (Kaunas) 2020;56:576. [Crossref] [PubMed]
- D'Angelo SP, Larkin J, Sosman JA, et al. Efficacy and Safety of Nivolumab Alone or in Combination With Ipilimumab in Patients With Mucosal Melanoma: A Pooled Analysis. J Clin Oncol 2017;35:226-35. [Crossref] [PubMed]
- Filippini A, Zorzi F, Bna' C, et al. Dark sputum: An atypical presentation of primary pulmonary malignant melanoma. Respir Med Case Rep 2015;15:118-20. [Crossref] [PubMed]
- Smith JL Jr, Stehlin JS Jr. Spontaneous regression of primary malignant melanomas with regional metastases. Cancer 1965;18:1399-415. [Crossref] [PubMed]
- Jensen OA, Egedorf J. Primary malignant melanoma of the lung. Scand J Respir Dis 1967;48:127-35. [PubMed]
- Allen MS Jr, Drash EC. Primary melanoma of the lung. Cancer 1968;21:154-9. [Crossref] [PubMed]
- Reed RJ 3rd, Kent EM. Solitary pulmonary melanomas: two case reports. J Thorac Cardiovasc Surg 1964;48:226-31. [Crossref] [PubMed]
- Jennings TA, Axiotis CA, Kress Y, et al. Primary malignant melanoma of the lower respiratory tract. Report of a case and literature review. Am J Clin Pathol 1990;94:649-55. [Crossref] [PubMed]
- Pan XD, Zhang B, Guo LC, et al. Primary malignant melanoma of the lung in the elderly: case report and literature review. Chin Med J (Engl) 2010;123:1815-7. [PubMed]
- Essner R, Lee JH, Wanek LA, et al. Contemporary surgical treatment of advanced-stage melanoma. Arch Surg 2004;139:961-6; discussion 966-7. [Crossref] [PubMed]
- Bastiaannet E, Beukema JC, Hoekstra HJ. Radiation therapy following lymph node dissection in melanoma patients: treatment, outcome and complications. Cancer Treat Rev 2005;31:18-26. [Crossref] [PubMed]
- Bajetta E, Del Vecchio M, Bernard-Marty C, et al. Metastatic melanoma: chemotherapy. Semin Oncol 2002;29:427-45. [Crossref] [PubMed]
- Ascierto PA, Minor D, Ribas A, et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol 2013;31:3205-11. [Crossref] [PubMed]
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367:107-14. [Crossref] [PubMed]
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol 2011;29:1239-46. [Crossref] [PubMed]
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33. [Crossref] [PubMed]
- Bernal L, Restrepo J, Alarcón ML, et al. Primary BRAF Mutant Melanoma of the Lung Treated with Immunotherapy and Pulmonary Bilobectomy: A Case Report. Am J Case Rep 2021;22:e927757. [Crossref] [PubMed]
- Shikuma K, Omasa M, Yutaka Y, et al. Treatment of primary melanoma of the lung monitored by 5-S-cysteinyldopa levels. Ann Thorac Surg 2009;87:1264-6. [Crossref] [PubMed]
Cite this article as: Xiao H, Lv X, Zhou S, Zhang Z, Wang X. Right upper lobectomy after immunotherapy for primary malignant melanoma of the lung: a case report and literature review. AME Case Rep 2024;8:5.


