A case report of giant gangliocytoma of mediastinum
Highlight box
Key findings
• In this report, we report a ganglioneuroma (GN) case. GN is a rare benign neurogenic tumor with no or only mild clinical symptoms. Preoperative imaging is of great help in the diagnosis and treatment of GN. Surgery is the treatment of choice for GN.
What is known and what is new?
• GN is a rare and easily misdiagnosed disease, many of which require pathological confirmation before diagnosis.
• In this report, we report a patient who was admitted to the hospital with a mediastinal tumor which is giant rarely.
What is the implication, and what should change now?
• A comprehensive diagnosis needs to be made using imaging examination before surgery.
Introduction
Tumors of sympathetic origin include ganglioneuroma (GN), ganglioblastoma, and neuroblastoma. GN, also known as ganglion cell tumor and ganglion cell neurofibroma, is a rare benign neurogenic tumor that originates from primitive neural spine cells and can appear in any part of the sympathetic chain formed by primitive neural spine cells (1-3). It ranges from the base of the skull to the pelvis, and is more common in the mediastinum, retroperitoneum, and adrenal glands (4-7). Most of the patients have no obvious symptoms clinically, and they are mostly found in the physical examination, which can easily lead to misdiagnosis and missed diagnosis. During surgery, the location of the tumor determines the difficulty of the operation, and most tumors are closely related to the surrounding tissue anatomy. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-55/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 30-year-old male patient was found to have a mediastinal mass 2 weeks ago during a routine examination. Through questioning the medical history, the patient occasionally had chest pain before 1 month, but it was tolerable and relieved spontaneously in a short period of time, without cough, expectoration, fever, night sweats, nausea, vomiting and other symptoms. Since the onset of the disease, the patient has been in good spirits, normal diet, and no significant changes in body weight. There is no special disease in the patient’s past-history. The patient’s height was 180 cm, weight was 59 kg, body mass index (BMI) =18.2 kg/m2. Physical examination showed no obvious positive signs. This patient has not received treatment before hospitalization.
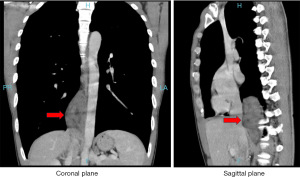
After the patient was hospitalized, enhanced chest computed tomography (CT) showed that mixed soft tissue density mass shadow was seen next to the mediastinal spine, and the CT value of plain scan was about 29–34 Hounsfield unit (HU), the enhancement was not significant, and the linear enhancement shadow was seen adjacent to the descending aorta, and the mass shadow was the largest. The diameter is about 58 mm × 31 mm. Consider the right mediastinum, paraspinal space-occupying lesions, and consider neurogenic tumors, pulmonary sequestration is not excluded, and malignant tumors are not excluded (Figure 1). In addition, positron emission tomography-CT (PET-CT) showed an opacity density mass in the right posterior mediastinum and increased metabolism. Considering the possibility of neurogenic tumor or teratoma, biopsy is recommended to confirm the pathology, and the possibility of malignant tumor is not excluded.
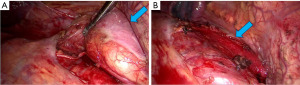
After completing the preoperative examination, the patient had no contraindications to surgery, that is, thoracoscopic tumor resection was performed. The tumor found during the operation was located on the lateral side of the lower esophagus under the inferior pulmonary ligament, behind the inferior vena cava, and in front of the spine. The tumor had a tough texture, and the outer side of the tumor was covered by a wall layer of pleural membrane. The tumor was loosely adhered to the surrounding organs and was freed by blunt-sharp combination. During the dissociation process, care should be taken to protect the esophagus, inferior vena cava, aorta, thoracic duct, and azygos vein, and expose the pedicle of the tumor. The tumor was completely resected, the specimen was removed, and sent for pathological examination (Figures 2,3).
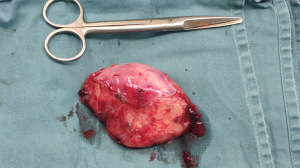
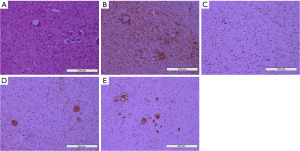
Postoperative pathology report described that the surgical specimen was a posterior mediastinal mass, which was one oval-shaped mass measuring 9.5 cm × 6.0 cm × 3.0 cm. Part of the surface of the mass was enveloped and smooth; the mass was dissected along the largest side of the mass, and the cut surface presented as a yellowish gray, grayish pink. The parenchymal mass was moderately hard in texture. Diagnosis was GN. Immunohistochemical results showed that neurofilament protein (+), neuron-specific enolase (+), S-100 (partial +), SOX-10 (+), chromogranin A (CgA) (+), Ki-67 (+1%), synaptophysin (+), CD34 (+), glial fibrillary acid protein (GFAP) (−), desmin (−), MyoD1 (−), CD117 (−), DOG1 (−) (Figure 4).
The surgery was successful, and the patient’s symptoms were completely relieved after the surgery. The patient underwent chest X-ray review at the 6th month after discharge, and no recurrence was found. The patient has fully recovered their health, there is no adverse and unanticipated events. The patient is very satisfied with the treatment process.
Timeline
During the 1st–7th days after hospitalization, laboratory examination and preoperative evaluation. The 8th day was operation day. The 9th–12th days were postoperative duration (Figure 5).
Discussion
GN is a rare benign neurogenic tumor originating from the sympathetic ganglia, mostly distributed along the paraspinal region, and the predilection sites are the posterior mediastinum, retroperitoneum, and adrenal glands (8,9). In this case, the tumor was located in the front of the spine, the posterior mediastinum was close to the diaphragm, and had a complete capsule, and the tumor was large.
The incidence of this disease is low, the tumor grows slowly, and often has no clinical symptoms or only mild clinical symptoms, and some only show compression symptoms when the tumor volume is large. A small number of patients showed corresponding symptoms due to the neuroendocrine function of the tumor. Rodriguez et al. rarer neurogenic tumors may also involve the mediastinum, a possibility that diagnostic pathologists and cytopathologists must be aware of given the diagnostic and therapeutic challenges that they present (10). The patient’s BMI was 18.2 kg/m2, which was rare in gangliocytoma. The patients with obesity were positively related to the gangliocytoma, especially in the adrenal GN (11,12). The patient in this case had no obvious clinical symptoms, and the lesion was discovered incidentally during the physical examination.
In the absence of a completely clear preoperative diagnosis, we believe that a preoperative puncture biopsy is necessary to be done. At present, because of the development of technology, even for malignant tumors, the chance of needle to metastasis due to fine needle puncture technique is very low, which is estimated to be about 0.003–0.009% (13). However, in this case, the patient’s ganglion cell neuroma was closely related to the surrounding tissues, closely related to the large blood vessels, and close to the thoracic duct, located above the celiac pond, which was a higher risk. Based on the imaging data, we considered the possibility of benign tumor in this disease. Therefore, we decided to perform surgical resection of the tumor.
Surgery is the treatment of choice for GN. Segars et al. consider GN can be treated with excision. Though these tumors are considered benign, due to the unclear nature of their development, surgical removal is recommended (14). However, attention should be paid to the anatomy of the tumor and surrounding tissues during the operation. The GN in our patient is closely related to the surrounding tissue, especially near the thoracic duct, above the cisterna chyli. Surgical removal of the tumor requires protection of the cisterna chyle, if it is damaged, it may lead to chylothorax and cause serious surgical complications. GNs are not sensitive to chemotherapy, and no chemotherapy is required after surgery, only total or subtotal tumor resection is required.
Conclusions
In conclusion, GN is a rare benign neurogenic tumor with no or only mild clinical symptoms. Enhanced CT is of great help in the diagnosis and treatment of GN. Surgery is the treatment of choice for GN. The difficulty of the operation lies in the location of the tumor and the anatomy of the surrounding tissue. The tumor should be carefully removed during the operation to prevent postoperative complications.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-55/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-55/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-55/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lonergan GJ, Schwab CM, Suarez ES, et al. Neuroblastoma, ganglioneuroblastoma, and ganglioneuroma: radiologic-pathologic correlation. Radiographics 2002;22:911-34. [Crossref] [PubMed]
- Angelini P, Baruchel S, Marrano P, et al. The neuroblastoma and ganglion components of nodular ganglioneuroblastoma are genetically similar: evidence against separate clonal origins. Mod Pathol 2015;28:166-76. [Crossref] [PubMed]
- Choi JH, Ro JY. Mediastinal neuroblastoma, ganglioneuroblastoma, and ganglioneuroma: Pathology review and diagnostic approach. Semin Diagn Pathol 2022;39:120-30. [Crossref] [PubMed]
- Goldberg JL, Tong J, McGrath LB Jr. Spinal Ganglioneuroma. World Neurosurg 2022;162:15-6. [Crossref] [PubMed]
- Alqahtani SM, Alshehri M, Adi H, et al. Left Adrenal Ganglioneuroma Treated by Laparoscopic Adrenalectomy in a 41-Year-Old Woman: A Case Report. Am J Case Rep 2022;23:e936138. [Crossref] [PubMed]
- Agarwal S, Wang Y, Iyer PG, et al. Incidental Colonic Ganglioneuroma on Surveillance Colonoscopy. ACG Case Rep J 2022;9:e00727. [Crossref] [PubMed]
- Aslan M, Dogukan FM. A Rare Cause of Dysphagia: A Giant Ganglioneuroma in Parapharyngeal Space. J Maxillofac Oral Surg 2022;21:99-101. [Crossref] [PubMed]
- Albonico G, Pellegrino G, Maisano M, et al. Ganglioneuroma of parapharyngeal region. Arch Pathol Lab Med 2001;125:1217-8. [Crossref] [PubMed]
- Aynaou H, Salhi H, El Ouahabi H. Adrenal Ganglioneuroma: A Case Report. Cureus 2022;14:e27634. [PubMed]
- Rodriguez EF, Jones R, Miller D, et al. Neurogenic Tumors of the Mediastinum. Semin Diagn Pathol 2020;37:179-86. [Crossref] [PubMed]
- Beal MF, Kleinman GM, Ojemann RG, et al. Gangliocytoma of third ventricle: hyperphagia, somnolence, and dementia. Neurology 1981;31:1224-8. [Crossref] [PubMed]
- Sandru F, Dumitrascu MC, Petca A, et al. Adrenal ganglioneuroma: Prognostic factors Exp Ther Med 2021;22:1338. (Review). [Crossref] [PubMed]
- Gao RY, Wu BH, Shen XY, et al. Overlooked risk for needle tract seeding following endoscopic ultrasound-guided minimally invasive tissue acquisition. World J Gastroenterol 2020;26:6182-94. [Crossref] [PubMed]
- Segars KA, Baltazar D, Baribault K, et al. Cutaneous ganglioneuroma: A case report and discussion of the literature. J Cutan Pathol 2019;46:293-6. [Crossref] [PubMed]
Cite this article as: Sheng Z, Tang J, Jiang Y, Du P, Chen S. A case report of giant gangliocytoma of mediastinum. AME Case Rep 2023;7:47.