
Fluorescence-guided surgery with indocyanine green to identify an idiopathic chyle leak—case report
Highlight box
Key findings
• Fluorescence-guided thoracic surgery using indocyanine green (ICG) can help localize the leakage site and help seal idiopathic chylothoraces.
What is known and what is new?
• The use of fluorescence-guided surgery to identify traumatic chylothoraces is well described.
• Non-traumatic chylothoraces remains more of diagnostic and therapeutic challenge.
• We demonstrate the use of ICG in fluorescence-guided surgery in a multidisciplinary setting with interventional radiology to localize and treat an idiopathic chylothrax.
What is the implication, and what should change now?
• Increased use of ICG in the treatment of refractory chyhlothoraces with a multidisciplinary approach might help localize treatment refractory disease.
Introduction
Chylothorax is leakage of chyle into the pleural space. It can happen either due to thoracic duct (TD) injury or without obvious trauma (non-traumatic). Most non-traumatic chylothoraxes are idiopathic (1). Chylothorax is associated with up to 50% morbidity due to nutritional losses, fluctuations in intravascular volumes, and respiratory compromise (2). Patients typically present with shortness of breath, chest heaviness, discomfort, or a cough. The initial work-up includes a chest X-ray, followed by thoracentesis and fluid analysis. Elevated triglyceride levels and a high percentage of lymphocytes on cell count confirm the diagnosis of a chyle leak.
Managements of chylothorax start with a conservative approach by diet modification. When this fails, we resort to tube thoracostomy. When tube thoracostomy fails, surgical TD ligation or TD embolization, is performed. Lymphangiography helps define the anatomy of the lymphatic channels and identify the site of leakage during TD embolization (3). Newer techniques such as indocyanine green (ICG) lymphography allow real-time delineation of anatomy and site of injury and has been described in identifying post-operative chylothoraxes (4). We describe below the use of ICG in a fluorescence-guided surgical approach to identify an idiopathic chylothorax that has been refractory to medical therapy and lymph duct embolization. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-53/rc).
Case presentation
A 50-year-old female with a recent history of coronavirus disease (COVID)-19, latent tuberculosis, anxiety, breast augmentation, and face lift surgery infection presented with shortness of breath. While traveling in August 2022, she developed right sided chest discomfort and a cough which was initially attributed to post-COVID symptoms. A chest X-ray ordered by her primary care physician revealed a significant right pleural effusion, and she was referred to the emergency room (ER) for further evaluation. She was admitted to the hospital for 2 weeks, where a chest tube was inserted and pleural fluid analysis confirmed the diagnosis of a chylothorax. Conservative management such as keeping her nil per os (NPO) and on total parenteral nutrition (TPN) were attempted but with little success with greater than 1 L of chest tube output per day.
She was transferred to our hospital for further management in September 2022. Initial magnetic resonance lymphangiogram (MRL) revealed abnormal lymphatic enhancements in the right paraspinal thoracic space (Figure 1A) as well as lympho-venous junction obstruction with large neck collaterals. The decision was made to perform percutaneous lympho-venous junction angioplasty with 5 mm balloon, that was unsuccessful to alleviate chylothorax.
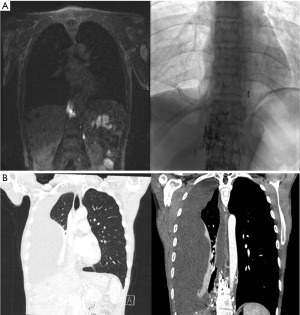
Consequently, she underwent percutaneous glue embolization of the paraspinal lymphatic enhancements without clinical improvement and hence was another unsuccessful embolization. Repeat MRL showed a patent TD and extravasation of contrast from the area of paraspinal enhancement. She underwent a third attempt of glue embolization on November 11, 2022, after which the leakage was smaller per the MRL of November 30, 2022. A few weeks later, she had worsening shortness of breath which prompted a computed tomography (CT) scan that showed a loculated effusion in her right lung and extensive lymphatic contrast in the paraspinal lymphatic system (Figure 1B). The decision was made to refer her to thoracic surgery for consideration of mechanical pleurodesis.
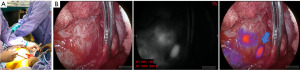
Given the location of the lesion and the concern of difficulty visualizing using thoracoscopic approaches, the decision was then made to proceed with a thoracotomy. Also, if we were to start the injection with ICG and were unable to immediately visualize the area thoracoscopically, the fluorescent chyle will then start pooling around the area of concern by the time we convert to a thoracotomy and we would lose the localization advantage. The goal was to identify the site of TD leakage and perform a mechanical pleurodesis assisted by intraoperative imaging. She underwent an 8th intercostal space posterolateral thoracotomy. This was followed by a lung pleurectomy and parietal decortication. The inferior pulmonary ligament was then divided. Attempts at instillation of heavy cream into the gastrointestinal tract were unable to localize an obvious site of leakage. Then, 10 mg (5 mg/kg) of ICG was injected into the right inguinal lymph nodes (Figure 2A). Using a camera capable of detection of near-infrared (NIR) light, we were able to visualize the site from which the ICG was extravasating in the chest (Figure 2B). Multiple clips were inserted in that area around the site from which fluorescent fluid was believed to emerge. Then, glue was injected in the area to further help in reducing the leak. A chest tube was placed, the chest cavity was closed and the procedure concluded.
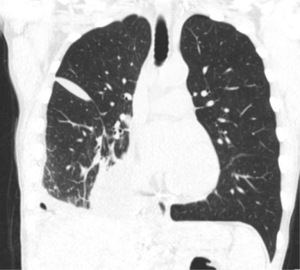
Over the next few days, the patient was kept NPO in order to reduce the lymphatic flow and facilitate the healing of the chyle leak. With decreasing chest tube output, her diet was advanced over the next 2 weeks to a clear liquid diet, full liquid diet and eventually a regular diet. However, she continued to have chylous outputs from her chest tubes. A CT scan showed a persistent pleural collection or her right side that was not drained by the chest tube in place. Meanwhile, a repeat MRL showed markedly decreased or resolved upper abdominal lymphatic leak into the right pleural space. A chest tube was placed to drain the loculated posterior effusion. Due to persistent residual output, she underwent a postoperative embolization of the lymphatic mass. Her chest tube output dropped significantly thereafter, and she was discharged home. A CT scan demonstrated no meaningful changes to her last scan prior to discharge (Figure 3). The chest tube was removed. Two weeks later, she was seen in clinic and reported significant improvement in her symptoms and returned to her baseline function. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Historically the non-traumatic chylothorax has been notoriously difficult to treat. The use of ICG for visualization of the TD during esophagectomy and cervical lymphadenectomy has been described and shown to decrease the likelihood of a TD injury and subsequent leak (3). In our study, we describe the use of ICG in fluorescence-guided surgery to diagnose and treat an idiopathic chylothorax (5).
The advantages of this technique are that both surgeons and interventional radiologist can be working at the same time. If there is need to inject more contrast, that can be easily done through the already established injection route. Also, the interventional radiologist may wish to introduce interventions under direct visualization intraoperatively with the help of intraoperative molecular imaging. Furthermore, in cases where multiple interventions done by interventional radiology (IR) are unsuccessful, direct visualization of anatomy might help in understanding the underlying disease process. This is especially worthwhile when patients are already undergoing a thoracotomy for other purposes such as pleurodesis in this case.
A major limitation in our patient’s refractory disease is the inability to detect the leak intraoperatively and even previously with the failed embolizations. From an IR standpoint, pedal lymphangiography is used to be used to help visualize lymphatics. Patients would receive contrast in the interdigital space that would allow for visualization of lymphatic anomalies. Because of the time needed to get opacification of the cisterna chyli and the TD, intranodal lymphangiography is used by accessing the inguinal nodes (6). In our approach, the patient’s groin was prepped and while the surgeon was working on gaining access to the site, the interventional radiologist was working on injecting ICG into the nodal basin.
The main limitation of this methodology is the fact that it entails multiple personnel and can be labor intensive. Although a limitation, it is also a strength as we get the viewpoint of multiple disciplines and a multidisciplinary approach to address a challenging diagnostic and therapeutic problem. This reinforces the importance of multidisciplinary management of this disease process.
Conclusions
We describe the use of fluorescence-guided surgery to localize and treat a nontraumatic idiopathic chylothorax while performing pleurodesis. The patient has had multiple embolization and glue embolization prior to this procedure but was only able to stop the fluid from reaccumulating when this procedure was combined with future IR embolization.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-53/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-53/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-53/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gurevich A, Hur S, Singhal S, et al. Nontraumatic Chylothorax and Chylopericardium: Diagnosis and Treatment Using an Algorithmic Approach Based on Novel Lymphatic Imaging. Ann Am Thorac Soc 2022;19:756-62. [Crossref] [PubMed]
- Agrawal A, Chaddha U, Kaul V, et al. Multidisciplinary Management of Chylothorax. Chest 2022;162:1402-12. [Crossref] [PubMed]
- Kaburagi T, Takeuchi H, Oyama T, et al. Intraoperative fluorescence lymphography using indocyanine green in a patient with chylothorax after esophagectomy: report of a case. Surg Today 2013;43:206-10. [Crossref] [PubMed]
- Bassi M, Vannucci J, Venuta F, et al. Effectiveness of indocyanine green fluorescence for the identification of thoracic duct in recurrent idiopathic chylothorax. Interact Cardiovasc Thorac Surg 2020;31:284. [Crossref] [PubMed]
- Pieper CC, Feisst A, Schild HH. Contrast-enhanced Interstitial Transpedal MR Lymphangiography for Thoracic Chylous Effusions. Radiology 2020;295:458-66. [Crossref] [PubMed]
- Patel S, Hur S, Khaddash T, et al. Intranodal CT Lymphangiography with Water-soluble Iodinated Contrast Medium for Imaging of the Central Lymphatic System. Radiology 2022;302:228-33. [Crossref] [PubMed]
Cite this article as: Bou-Samra P, Chang A, Singhal S, Itkin M. Fluorescence-guided surgery with indocyanine green to identify an idiopathic chyle leak—case report. AME Case Rep 2023;7:41.


