
Multiple cerebral septic emboli sourcing from a ventricular assist device: a case report
Highlight box
Key findings
• The case report describes a patient with a ventricular assist device, suffering from heart failure and awaiting for heart transplant. He presented with a right hemiparesis and fever. Brain CT scan uncovered embolic foci. Two major Duke’s criteria (blood culture and echocardiographic findings) were positive.
What is known and what is new?
• Patients with implantable cardiac devices can present with systemic complications. While being anticoagulated, hemorrhagic events may happen, even under strict monitoring.
• LVAD associated infections such as endocarditis may complicate an already difficult general condition. Particular precautions are necessary, for a prompt diagnosis, and focused therapies.
What is the implication, and what should change now?
• The neurological conditions that accompany high-risk cardiac patients are various. A greater awareness of clinical pictures is important. Studies suggest that resumption of anticoagulation therapy should start after a cerebral hemorrhagic event, once the active bleeding has stopped.
Introduction
Infective endocarditis (IE) is a disease of the endocardial surface of the heart, degenerative valvulopathies or prosthetic heart valves, and cardiovascular devices, caused by a variety of infectious microorganisms, which remains a major clinical problem due to neurological complications occurring during the active course of it. A broad spectrum of neurological complications are observed such as nonfocal encephalopathy, headache, seizures, meningitis, brain abscess, mycotic aneurysms, ischemic stroke and cerebral hemorrhage. The annual incidence of IE is reported to be 3 to 10 per 100,000 people and in 20–40% of them neurological complications are observed (1-3). We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-50/rc).
Case presentation
We present a case of a 46-year-old male admitted to our emergency room department with a 3-day history of tiredness, high fever with shivering, headache and profuse sweating. The patient’s past medical history includes hypertension, cardiac failure, paroxysmal atrial fibrillation. It has been two and half years since he has been carrying a left ventricular assist device [HeartMate II® CF-LVAD (Thoratec Corporation, Pleasanton, CA, USA)] and an implantable cardioverter defibrillator (ICD). Besides heart failure medications, the treatment included a vitamin K antagonist oral anticoagulant (acenocumarol) with an international normalized ratio (INR) dependent dosage.
Upon physical examination, a copious purulent drainage was noticed in the driveline exit site of left ventricular assist device (LVAD). Vital parameters revealed slight hypotension (90/60 mmHg) with a mean arterial pressure of 70 mmHg, a heart rate with 78 heartbeats per minute with frequent ventricular extrasystoles, low respiratory frequency (8/minute), subfebrile temperature up to 37.7 ℃ and normal oxygen saturation (97%).
A complete blood count revealed elevated white blood cells (WBCs) 15.84×103/µL (normal range, 4.0×103–10.5×103/µL), of which 83.30% neutrophils (normal range, 42.0–72.0%), moderate microcytic anemia; hemoglobin 10.7 g/dL (normal range, 13.0–16.5 g/dL), hematocrit 31.10% (range, 40.0–50.0%), mean corpuscular volume (MCV) 71.20 fL (range, 78.2–97.9 fL), mean corpuscular hemoglobin (MCH) 24.6 pg (range, 25.0–33.0 pg), while urine test resulted normal. High C-reactive protein 12.48 mg/dL (range, 0–0.5 mg/dL) and high procalcitonin 27.70 ng/mL (range, 0–0.5 ng/mL) raised the suspicion of sepsis and the patient was immediately hospitalized.
Two blood cultures 60 minutes apart from each other were recommended for collection when the patient’s body temperature reached more than 38 ℃. At 3 hours following admission, the patient’s speech became slurred and a motor deficit with motor force of 3/5 of right upper limb was noticed. An urgent head computerized tomography (CT) scan was performed which showed multiple cortical lesions in left frontal lobe and Rolandic gyrus of left parietal lobe, a small hemorrhagic lesion in left occipital lobe with minimal perifocal edema, another lesion in the posterior parietal lobe with perifocal edema and an ischemic lesion in right occipital lobe too (Figure 1). Blood coagulation tests showed an increased INR of 3.80. Later that day he became febrile up to 39.0 ℃ so that a blood sample was taken for culture testing as well as a sterile aspirate from exit site of LVAD. Anticoagulation therapy stopped immediately, and empirical intravenous antibiotic therapy was started (cefuroxime 750 mg every 6 hours). The hemoculture yielded a Staphylococcus epidermidis methicillin-resistant, and therapy was modified to intravenous vancomycin 30 mg/kg for 4 consecutive weeks.
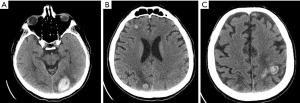
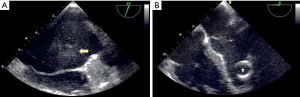
The following day the patient performed a thoraco-abdominal CT scan, which noticed two enlarged reactive paratracheal lymph nodes, with no other relevant radiographic findings. While the patient was becoming more lethargic and with continuous fever regardless of antipyretics, he experienced a motor focal seizure that lasted 20 seconds, therefore he was set under therapy with intravenous levetiracetam 1,000 mg/day twice a day. INR was measured again and the result was at 3.93. Knowing the fact that sepsis can lead to disseminated intravascular coagulation by consuming coagulation factors, transfusion of fresh frozen plasma (FFP) was applied. The body temperature became intermittent and a slow improvement was seen in the patient’s symptoms until the next day when the patient complained a sudden vision impairment. Neurological examination revealed right homonymous lateral hemianopia, right central facial palsy, and right upper limb monoparesis with motor force of 2/5. Another head CT scan was performed and an enlargement of left occipital lesion was noticed, with two other small new hemorrhagic lesions, in right frontal and temporal lobes, 7 and 3 mm respectively. Another unit of FFP transfusion was applied. Transthoracic and trans-esophageal echocardiogram were also performed and it was seen a thrombus in left atrium, moderate mitral and aortal regurgitation as well as left atrial enlargement (Figure 2).
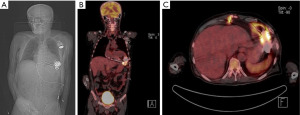
Further, the patient performed a positron emission tomography (PET)-CT scan examination, which showed LVAD infection and confirmed it as the source of septic emboli (Figure 3).
At the seventh day of admission, prophylactic dosage of subcutaneous enoxaparin was started (40 mg/day), and it was switched to therapeutic dosage at day 15 (1.5 mg/kg/day). The following days, all the laboratory findings and patient’s symptoms showed significant improvement; he became afebrile, WBC count lowered to 13.40×103/µL, C-reactive protein 7.19 mg/dL, procalcitonin 0.42 ng/mL and INR value was 1.33. Due to repetitive aware focal motor seizures, the patient had an electroencephalogram, which showed low amplitude alpha rhythm with a frequency at 8–10 Hz, left temporal and bilateral occipital complex discharges. Intravenous levetiracetam was switched to oral administration 1,000 mg/d twice a day. The final head CT scan showed resorption of right frontal and occipital hemorrhagic lesions, smaller left parietal and frontal lesions, while the left parieto-occipital hemorrhagic lesion seemed the same size and with perifocal edema.
He was transferred to a cardiac surgery facility for a revision. Eventually, the ventricular assist device (VAD) was replaced under general anesthesia and the patient remained in the waiting list for heart transplantation.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The number of patients suffering from heart failure has increased lately and they often require heart transplantation for survival. However, the number of heart transplantation procedures remains almost the same due to a shortage in organ donors. Therefore, a temporarily option until a heart transplant can be done, is using mechanical circulatory support devices and in most cases LVAD (4).
LVADs have played a crucial role in the treatment of advanced heart failure patients, but considering the fact that they are a foreign body and they communicate with the outer environment through an exit driveline percutaneously, they still carry the risk of infection (5). According to the International Society for Heart and Lung Transplantation (ISHLT), LVAD infection is an infection that occurs when having a LVAD that may or may not be attributable to the LVAD, but that may need special consideration if an LVAD is in place. Besides those directly associated with the device, several types of infections such as catheter-related bloodstream infection or bacteremia are included in this definition (6).
Sixty percent of all patients undergoing LVAD are reported to have developed infection including bloodstream infection, sepsis, and endocarditis (7). LVAD endocarditis, just like prosthetic valve endocarditis, can lead to a variety of complications such as LVAD dysfunction, LVAD thrombosis and septic embolization (8). Specific diagnostic criteria for suspected IE have been proposed since 1994 and are largely applied (9). The Duke’s criteria in fact, are a composed set of “major criteria” (typical blood culture and positive echocardiogram) and “minor criteria” (predisposition, fever, as well as suggestive echocardiogram and microbiologic findings, among others).
Stroke is the most common neurological complication caused by IE, affecting up to 35% of all patients. Hemorrhagic stroke is seen in nearly 20% of patients with cerebrovascular complications of IE, and its causes include hemorrhagic transformations of ischemic lesions, rupture of mycotic aneurysms or vessel wall inflammation due to septic necrotic arteritis (1,10).
After a stroke has occurred and cerebral hemorrhage has been witnessed in CT scan, cardiac surgery timing should be re-considered (2). Actual guidelines suggest that cardiac surgery should be delayed by 4 weeks, after cerebral hemorrhage in IE.
For the reversal of INR in a situation of active bleeding, we could choose between two options, FFP or vitamin K administration. Prothrombin complex concentrate is another option of first line medication in treating over-warfarinization. Due to the fact that vitamin K, vital for the process of synthesis of new coagulation proteins, will take up to 6 hours to start working and its effect will fully manifest after 24 hours, FFP seems to be the option of choice (11). In addition, when having a concomitant diagnosis of sepsis, FFP is also preferable because of its important clinical effects such as volume expansion and correction of abnormal coagulation tests (12). Guidelines for the reversal of oral anticoagulants in acute intracerebral hemorrhage are available, and must be accordingly implemented (13).
Conclusions
Patients with a high cardiac risk might present a diversity of neurological conditions. A greater awareness and prompt intervention is needed for such complications to be successfully treated.
Studies have shown that resumption of prophylactic anticoagulation therapy should start after a cerebral hemorrhagic event, but only after the situation of active bleeding is put under control. In our case, we decided to re-start it after 2 weeks while being sure that hematoma resorption was effective. This was also because the patient was carrying a mechanical device (LVAD) which has a high risk of forming clots and systemically pumping them might cause remote ischemic events (14,15).
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-50/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-50/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-50/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sotero FD, Rosário M, Fonseca AC, et al. Neurological Complications of Infective Endocarditis. Curr Neurol Neurosci Rep 2019;19:23. [Crossref] [PubMed]
- Scheggi V, Menale S, Tonietti B, et al. Impact of septic cerebral embolism on prognosis and therapeutic strategies of infective endocarditis: a retrospective study in a surgical centre. BMC Infect Dis 2022;22:554. [Crossref] [PubMed]
- Chen H, Zhan Y, Zhang K, et al. The Global, Regional, and National Burden and Trends of Infective Endocarditis From 1990 to 2019: Results From the Global Burden of Disease Study 2019. Front Med (Lausanne) 2022;9:774224. [Crossref] [PubMed]
- Toyoda Y, Guy TS, Kashem A. Present status and future perspectives of heart transplantation. Circ J 2013;77:1097-110. [Crossref] [PubMed]
- Thyagarajan B, Kumar MP, Sikachi RR, et al. Endocarditis in left ventricular assist device. Intractable Rare Dis Res 2016;5:177-84. [Crossref] [PubMed]
- O'Horo JC, Abu Saleh OM, Stulak JM, et al. Left Ventricular Assist Device Infections: A Systematic Review. ASAIO J 2018;64:287-94. [Crossref] [PubMed]
- Koval CE, Rakita R, Infectious Diseases AST. Community of Practice. Ventricular assist device related infections and solid organ transplantation. Am J Transplant 2013;13:348-54. [Crossref] [PubMed]
- Argenziano M, Catanese KA, Moazami N, et al. The influence of infection on survival and successful transplantation in patients with left ventricular assist devices. J Heart Lung Transplant 1997;16:822-31. [PubMed]
- Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 1994;96:200-9. [Crossref] [PubMed]
- Jiad E, Gill SK, Krutikov M, et al. When the heart rules the head: ischaemic stroke and intracerebral haemorrhage complicating infective endocarditis. Pract Neurol 2017;17:28-34. [Crossref] [PubMed]
- Garcia DA, Crowther MA. Reversal of warfarin: case-based practice recommendations. Circulation 2012;125:2944-7. [Crossref] [PubMed]
- Qin X, Zhang W, Zhu X, et al. Early Fresh Frozen Plasma Transfusion: Is It Associated With Improved Outcomes of Patients With Sepsis? Front Med (Lausanne) 2021;8:754859. [Crossref] [PubMed]
- Christensen H, Cordonnier C, Kõrv J, et al. European Stroke Organisation Guideline on Reversal of Oral Anticoagulants in Acute Intracerebral Haemorrhage. Eur Stroke J 2019;4:294-306. [Crossref] [PubMed]
- Moon JY, Bae GH, Jung J, et al. Restarting anticoagulant therapy after intracranial hemorrhage in patients with atrial fibrillation: A nationwide retrospective cohort study. Int J Cardiol Heart Vasc 2022;40:101037. [Crossref] [PubMed]
- Lin SY, Chang YC, Lin FJ, et al. Post-Intracranial Hemorrhage Antithrombotic Therapy in Patients With Atrial Fibrillation. J Am Heart Assoc 2022;11:e022849. [Crossref] [PubMed]
Cite this article as: Roçi E, Puca E, Sula F, Dodaj S, Vyshka G. Multiple cerebral septic emboli sourcing from a ventricular assist device: a case report. AME Case Rep 2023;7:38.


