
Co-existing pericardial and pleural malignant mesothelioma responding well to nedaplatin and pemetrexed: a case report
Highlight box
Key findings
• This is the first case of co-existing pericardial and pleural malignant mesothelioma (MM) treated with nedaplatin and pemetrexed and responding well.
What is known and what is new?
• The current first-line chemotherapy for advanced MM is a combination with cisplatin and pemetrexed. However, nedaplatin is confirmed that it has the similar therapeutic effects as cisplatin but lower toxicity and higher water solubility in several carcinoma.
• Our case report provides evidence for the effective treatment of MM with nedaplatin and pemetrexed.
What is the implication, and what should change now?
• We suggest that chemotherapy combined nedaplatin with pemetrexed may be a more appropriate treatment in advanced MM. Further clinical trials which focus on the comparison of efficacy and toxicity between nedaplatin and cisplatin in treating MM are warrant.
Introduction
Background
Malignant mesothelioma (MM) is a rare cancer with a dismal prognosis that arises from mesothelial cells of the pleura (65–70%), peritoneum (10–20%), pericardium (1%) or tunica vaginalis (1%) (1,2). The insidious and nonspecific initial presentations with the low sensitivity of diagnostic methods make it hard to diagnose promptly and result in very poor prognosis. It is less common that two serosal cavities are both involved when the patient seeks medical attention firstly.
Rationale and knowledge gap
The current first-line chemotherapy for advanced MM is a combination with cisplatin and pemetrexed (3,4). However, cisplatin may cause some serious adverse effects, such as severe gastrointestinal side effects, renal toxic effects and so on, which always force patients to discontinue chemotherapy (5-7). Several clinical trials have found out that nedaplatin, a second-generation platinum-based antitumor agent, has the similar therapeutic effects as cisplatin but higher water solubility and lower toxicity (lower gastrointestinal toxicity and nephrotoxicity compared with cisplatin), which may be a more appropriate choice and used increasingly in chemotherapy of lung cancer (7,8). Moreover, the same clinical findings could be also confirmed in other carcinoma like nasopharyngeal carcinoma and esophageal cancer, in comparison between nedaplatin-based and cisplatin-based chemotherapy (9,10).
Objective
To our knowledge, there is no report of co-existing pericardial and pleural MM treated with nedaplatin and pemetrexed. Here, we report a 33-year-old woman diagnosed with co-existing pericardial and pleural MM is responding well to chemotherapy with nedaplatin and pemetrexed. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-102/rc).
Case presentation
The case timeline and treatment were summarized in Figure 1A. The whole dynamic changes in chest computed tomography (CT) imaging were shown in Figure 1B. A 33-year-old previously healthy woman who suffered from dyspnea and chest tightness for 6 days was hospitalized in Department of Cardiology in March 2020. She had worked in a kiln for more than 10 years which showed a history of occupational exposure to asbestos. Physical examination revealed a blood pressure of 123/87 mmHg, distended right jugular vein and both pulmonary rales. Chest CT showed a massive pericardial effusion, little pleural effusion and irregular interlobular fissure thickening (Figure 1B, panel I). Echocardiography showed no definite pericardial thickening or heart failure (ejection fraction 66%). Laboratory tests showed mild inflammation (C-reactive protein: 17.5 mg/L, procalcitonin: 0.061 ng/mL) and elevated concentration of cytokeratin fraction 21-1 (CYFRA 21-1; 5.11 ng/mL, baseline 3.3 ng/mL) and carbohydrate antigen-125 (CA125; 224.9 U/mL, baseline 35 U/mL) but not carcinoembryonic antigen (CEA). Emergency pericardiocentesis was performed and 1,860 mL of fluid was drained. Pericardial fluid was bloody and exudate with elevated lactate dehydrogenase (LDH; 6,954 U/L). The acid-fast bacilli stain was negative. Pericardial tuberculosis infection T cell spot test (T-SPOT.TB) was positive [the ratio of TB-specific antigen (TBAg) to phytohaemagglutinin (PHA), TBAg/PHA ratio: 0.11], but T-SPOT.TB in peripheral blood was negative. Cytological examination showed moderate mesothelial hyperplasia, but was negative for malignancy. Further positron-emission tomography (PET) revealed partial inflammation responses as the only manifestation. Thus, the patient was treated for suspected tuberculous pericarditis and received 6 months antituberculosis treatment (rifampicin 450 mg q.d., isoniazide 300 mg q.d., pyrazinamide 500 mg t.i.d., ethambutol 750 mg q.d.).
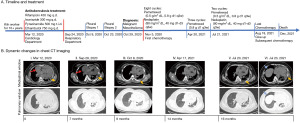
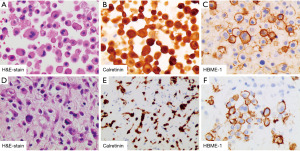
In September 2020, she was readmitted to respiratory apartment with 14 days history of cough, expectoration, dyspnea and chest tightness. Chest CT images showed massive pleural and pericardial effusion (Figure 1B, panel II). Thoracentesis and pericardiocentesis were performed. 3,300 mL of yellow pleural fluid and 1,910 mL of hemorrhagic pericardial fluid were drained. LDH was found to be elevated in both fluids, 5,372 U/L in pleural fluid and 1,818 U/L in pericardial fluid respectively. Both fluids showed marked mesothelial proliferation (Figure 2A). The mesothelial cells in pericardial fluid were strongly positive for calretinin, hector battifora mesothelial-1 (HBME-1), cytokeratin (CK) 5/6 but negative for thyroid transcription factor-1 (TTF-1), MOC31 immunohistochemically (Figure 2B,2C). Blood laboratory examination showed a normal concentration of CEA but up-regulated level of CYFRA 21-1 (31.84 ng/mL) and CA125 (299.3 U/mL). Second chest CT revealed an uneven left pleural thickening and thus twice left pleural biopsy were done (Figure 1B, panel III). Hematoxylin and eosin (H&E)-stained sections of the pleura showed a neoplasm characterized by a predominantly diffuse growth pattern and monomorphic epithelioid cells with eccentric, hyperchromatic nuclei (Figure 2D). The neoplastic cells were positive for calretinin, HBME-1, pan-cytokeratin (pan-CK), and vimentin (Figure 2E,2F). Immunostains for glucose transporter-1 (GLUT-1), Desmin, S100, Myogenin, cluster of differentiation (CD)34, B-cell lymphoma (BcL)-2 and Signal Transducer and Activator of Transcription-6 (STAT-6) were negative. She disagreed pericardial biopsy for its high risk.
MM (T4N2Mx, grade IV) in pericardium and pleural was diagnosed according to International Mesothelioma Interest Group (IMIG) staging system, based on the summarized results: (I) a long history of occupational exposure to asbestos; (II) clinical manifestation: cough, expectoration, dyspnea and chest tightness; (III) imaging results: recurrent pleural and pericardial effusion, pleural thickening; (IV) laboratory results: exudative effusion with significantly elevated LDH and marked mesothelial proliferation; high level of CYFRA 21-1 and CA125 but not CEA. (V) Immunohistochemical results: positive for calretinin, HBME-1, pan-CK, CK5/6, but negative for TTF-1, GLUT-1, Desmin, S100, Myogenin, CD34, BcL-2 and STAT-6. Because of the advanced carcinoma, palliative chemotherapy was chosen as the initial treatment. After 8 cycles of pemetrexed (0.5 g/m2 dL, 0.8 g d1 q3w) and nedaplatin (80 mg/m2 dL, 40 mg d1–d3 q3w) during 6 months, the clinical manifestations such as cough, expectoration, dyspnea and chest tightness were all alleviated significantly and the malignancy had been improved gradually without bone marrow toxicity, and maintained clinical stabilization based on non-recurrent massive pericardial or pleural effusion (Figure 1B, panel IV). Pemetrexed (0.8 g d1 q3w) was selected for subsequent maintenance therapy. However, the pleural thickening and pleural or pericardial fluid was increased after 3 cycles of pemetrexed (Figure 1B, panels V and VI). With another 2 cycles of pemetrexed and nedaplatin, she gave up chemotherapy due to the poor economic condition in August and died at home in December 2021. From the onset symptoms, the patient has survived for 22 months.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made.
Discussion
Key findings
To our knowledge, this is the first report of co-existing pericardial and pleural MM treated with nedaplatin and pemetrexed.
Strengths and limitations
In our case, although the patient refused subsequent chemotherapy because of poor economics, she responded well to nedaplatin and pemetrexed without bone marrow toxicity and didn’t have recurrent massive pericardial or pleural effusion again during chemotherapy. She had a total survival of 22 months after first clinical manifestation. However, the case report had a major limitation that we failed to obtain pericardial pathology because the patient disagreed pericardial biopsy for its high risk, although pericardial effusion cytology and pleural biopsy were all suggestive of MM.
Comparison with similar researches and explanations of findings
Pleural MM is the most common primary MM. Disease progression is seen usually by invading local contiguous tissue such as lung, whereas in rare instances pericardium and myocardium. Its common images show pleural effusion, diffuse pleural thickening, pulmonary nodules, lung encasement, mediastinal shift and elevation of hemidiaphragm (11). Primary pericardial MM is extremely infrequent tumor with the prevalence of 0.001–0.007% among cardiac tumors (12). Metastases to heart and pericardium are much more frequent than primary malignancy (13). Pericardial MM always presents with pericardial effusion, constrictive pericarditis, cardiac tamponade and heart failure (14). The diagnostic methods include cytology, biopsy, CT, magnetic resonance imaging (MRI), PET and ultrasonography. It is known that the diagnostic sensitivity of cytology is very low as only 20% (15). PET is often difficult to have positive manifestations in the early stage of MM and generally used to evaluate the metastatic extent and treatment efficacy assessment (16). Together with the insidious and nonspecific initial presentations, the low sensitivity of diagnostic methods makes it hard to diagnose promptly and results in very poor prognosis. Interestingly, in our case, both pericardium and pleura appeared to involved by mesothelioma. At the first medical visit, mild irregular interlobular fissure thickening with little pleural effusion and pericardial effusion without pericardial thickening were both presented synchronously, although PET didn’t reveal significant strong uptake which suggesting malignancy. Regrettably, a pleural or pericardial biopsy was not performed at that time. At the second visit after 6 months, pericardial effusion cytology and pleural biopsy were all suggestive of MM. The major limitation was that we failed to obtain pericardial pathology because the patient disagreed pericardial biopsy for its high risk. There was no clinical evidence of which malignancy was the primary one because it was hard to distinguish based on molecular marker. In terms of incidence, the greatest possibility is suspected primary pleural MM with pericardial metastasis. In rarer cases, primary MM coexisting in the pericardium and pleura might be suspected because the patient had long history of occupational asbestos exposure. Therefore, unresolving recurrent pleural and pericardial effusion should raise suspicion for MM.
The treatments of MM include surgical therapy, chemotherapy and radiotherapy (17). Surgical therapy is useful for localized disease, tumor reduction and preventing fatal tumor complications such as cardiac tamponade. However, when diagnosed, MM is often locally progressive or distant metastatic which is unavailable for surgery and should be administered by chemotherapy. No reliable large clinical trials for chemotherapy have been conducted. Therefore, a standard chemotherapeutic guideline has not yet been established. The current first-line chemotherapy is a combination with pemetrexed and cisplatin, which was confirmed to lead to longer survival times than cisplatin monotherapy (3,4). However, cisplatin and carboplatin may cause some serious adverse effects, such as myelosuppression, neurotoxicity and gastrointestinal reactions, which limit their clinical use and force to discontinue cisplatin-based chemotherapy (5-7). Nedaplatin, a second-generation platinum-based antitumor agent, has the similar therapeutic effects as cisplatin but higher water solubility, lower gastrointestinal toxicity and lower nephrotoxicity compared with cisplatin in lung cancer, nasopharyngeal carcinoma and esophageal cancer (7,9,10,18). Lin et al. and Zhong et al. reported that the efficacy of nedaplatin was similar as cisplatin but nedaplatin had higher tolerance and less toxicity, in treating advanced lung adenocarcinoma or cancer-induced pleural effusion (19,20).
Implications and actions needed
In terms of clinical tolerance and less adverse reactions, we suggest that chemotherapy of nedaplatin with pemetrexed may be a more appropriate treatment in advanced MM. Nevertheless, since it is only a case report, further clinical trials are warrant to analyze the efficacy, safety, dose and cycle required for maximal response and minimal toxicity, progressive-free survival and overall survival of nedaplatin plus pemetrexed in advanced MM.
Conclusions
MM is a rare cancer and a huge socioeconomic burden. Early recognition, staging and response evaluation are very critical to determine treatment. Chemotherapy combined nedaplatin with pemetrexed is effective for advanced MM with low toxicity and high tolerance. However, further clinical trials are warrant.
Acknowledgments
Funding: This work was supported by the grant from the Shantou Medical Health Science and Technology Plan (No. 190917085269841).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-102/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-22-102/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-102/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kralstein J, Frishman WH. Malignant pericardial diseases: diagnosis and treatment. Cardiol Clin 1987;5:583-9. [Crossref] [PubMed]
- Churg A, Cagle PT, Roggli VL. Diffuse malignant tumors of the serosal membranes. In: Tumors of the serosal membranes, 4th series. American Registry of Pathology; 2006:33-72.
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III Study of Pemetrexed in Combination With Cisplatin Versus Cisplatin Alone in Patients With Malignant Pleural Mesothelioma. J Clin Oncol 2023;41:2125-33. [Crossref] [PubMed]
- Ziółkowska B, Cybulska-Stopa B, Papantoniou D, et al. Systemic treatment in patients with malignant pleural mesothelioma - real life experience. BMC Cancer 2022;22:432. [Crossref] [PubMed]
- Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int 2014;2014:967826. [Crossref] [PubMed]
- Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol 2003;23:460-4. [Crossref] [PubMed]
- Tsvetkova D, Ivanova S. Application of Approved Cisplatin Derivatives in Combination Therapy against Different Cancer Diseases. Molecules 2022;27:2466. [Crossref] [PubMed]
- Liu Y, Yu S, Liu S, et al. Comparison of nedaplatin-based versus cisplatin-based chemotherapy for advanced non-small cell lung cancer among East Asian populations: A meta-analysis. Sci Rep 2015;5:10516. [Crossref] [PubMed]
- Zhang F, Wang Y, Wang ZQ, et al. Efficacy and safety of cisplatin-based versus nedaplatin-based regimens for the treatment of metastatic/recurrent and advanced esophageal squamous cell carcinoma: a systematic review and meta-analysis. Dis Esophagus 2017;30:1-8. [PubMed]
- Tang C, Wu F, Wang R, et al. Comparison between nedaplatin and cisplatin plus docetaxel combined with intensity-modulated radiotherapy for locoregionally advanced nasopharyngeal carcinoma: a multicenter randomized phase II clinical trial. Am J Cancer Res 2016;6:2064-75. [PubMed]
- Rossi G, Davoli F, Poletti V, et al. When the Diagnosis of Mesothelioma Challenges Textbooks and Guidelines. J Clin Med 2021;10:2434. [Crossref] [PubMed]
- Barroso AS, Leite S, Friões F, et al. Pericardial mesothelioma presenting as a suspected ST-elevation myocardial infarction. Rev Port Cardiol 2017;36:307.e1-5. [Crossref] [PubMed]
- Bussani R, De-Giorgio F, Abbate A, et al. Cardiac metastases. J Clin Pathol 2007;60:27-34. [Crossref] [PubMed]
- Song G, Bi W, Zhang X, et al. Localized primary malignant pericardial mesothelioma. J Clin Ultrasound 2019;47:178-81. [Crossref] [PubMed]
- Nilsson A, Rasmuson T. Primary Pericardial Mesothelioma: Report of a Patient and Literature Review. Case Rep Oncol 2009;2:125-32. [Crossref] [PubMed]
- Fennell DA, Gaudino G, O'Byrne KJ, et al. Advances in the systemic therapy of malignant pleural mesothelioma. Nat Clin Pract Oncol 2008;5:136-47. [Crossref] [PubMed]
- Hajj GNM, Cavarson CH, Pinto CAL, et al. Malignant pleural mesothelioma: an update. J Bras Pneumol 2021;47:e20210129. [PubMed]
- Kurata T, Tamura K, Yamamoto N, et al. Combination phase I study of nedaplatin and gemcitabine for advanced non-small-cell lung cancer. Br J Cancer 2004;90:2092-6. [Crossref] [PubMed]
- Lin Z, Lv WZ, Wang SY, et al. Efficacy and safety of pemetrexed and nedaplatin followed by pemetrexed maintenance therapy in advanced lung adenocarcinoma. Cancer Manag Res 2017;9:671-7. [Crossref] [PubMed]
- Zhong LZ, Xu HY, Zhao ZM, et al. Comparison of efficacy and toxicity between nedaplatin and cisplatin in treating malignant pleural effusion. Onco Targets Ther 2018;11:5509-12. [Crossref] [PubMed]
Cite this article as: Wu M, Li Z, Cai J, Zhong X, Zheng W, Wu S, Lin M, Zhang Q. Co-existing pericardial and pleural malignant mesothelioma responding well to nedaplatin and pemetrexed: a case report. AME Case Rep 2023;7:32.


