
Surgical treatment of neonatal Cantrell pentalogy: a case report and literature review
Highlight box
Key findings
• Surgery is an effective treatment method for Cantrell’s pentalogy, even if for low weight newborn.
What is known and what is new?
• Cantrell’s pentalogy is a congenital multiple malformation, which present a distinctive challenge for care-providers and surgeons.
• We report a new born twin with Cantrell’s pentalogy, who had three surgical operations in the neonatal period, and recovered smoothly.
What is the implication, and what should change now?
• Though Cantrell’s pentalogy is a rare and complex, surgery is an effective alternative, and its prognosis is related to the complexity of malformations.
Introduction
Pentalogy of Cantrell (PC) is a collection of hereditary malformations invoking the heart, pericardium, diaphragm, sternum, and abdominal wall (1). This disease is complicated in condition, low in survival rate and difficult to treat. Recently, a PC patient was admitted to the Children’s Hospital of Soochow University. Three surgical operations were performed during the neonatal period, and all deformities were corrected with satisfactory results. We took some photographs of this patient before and after surgery (Figure 1). We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-14/rc).
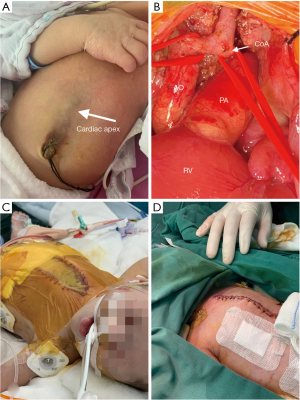
Case presentation
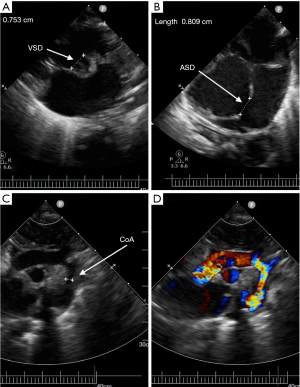
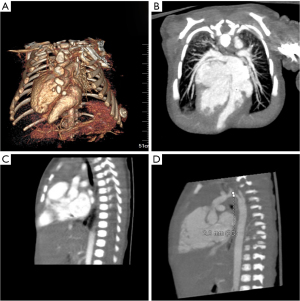
The male baby was G1P2, the younger fraternal twin, prenatal echocardiography indicated that he suffered from ventricular septal defect (VSD), atrial septal defect (ASD) and aortic arch hypoplasia. Due to the influence of twins, the structure of anterior abdominal wall is unclear in prenatal abdominal ultrasound. He was born at 37+1 W, with a weight of 2,400 g and an Apgar score of 10. His blood pressure of right upper limb was 75/35 mmHg, and 58/30 mmHg at lower limb. Oxygen saturations of upper and lower limb were 98% and 92% respectively. The lower end of sternum is suspiciously missing, the apex beating point is located at the lower edge of the median xiphoid process of sternum, and the upper abdominal muscle below the beating part is defective, the skin is still intact and in dark red, and a cord shape seems to be connected with the umbilicus wheel (Video 1, Figure 1A). Heart sounds are still strong, and IV/VI grade heart murmurs can be heard. Echocardiogram and cardiac CT angiography showed: dextroversion of the heart and coarctation of the aorta with arch hypoplasia, VSD, ASD, patent ductus arteriosus (PDA) and persistent left superior vena cava (Figure 2). Chest CT and 3D reconstruction showed incomplete ossification of the sternum, and the lower end of sternum was missing (Figure 3).
Prostaglandin was given to keep the PDA open after admission, and ventilation support was employed 9 days after birth.
On the 12th day after birth, the first operation was performed, including end-to-side anastomosis of aortic arch, patch repair of VSD, repair of ASD and ligation of PDA under deep hypothermic circulatory arrest (DHCA) and selective anterograde cerebral perfusion. During the operation, it was found that the lower sternum was defective, the rectus abdominis muscle was defective, and the diaphragm was intact, the attachment point of its front edge moved down to the navel, and the anterior lower wall of parietal pericardium was defective, forming a capsular bag with apex adhered. Aortic arch hypoplasia, the diameter of proximal and distal of the aortic arch were approximately 3–4 mm, the isthmus of aorta was obviously narrowed, with an inner diameter of only 1–2 mm (Figure 1B). The PDA was almost closed. The bilateral superior vena cava with small diameter. The diameter of the perimembranous VSD was 8 mm, and ASD was 8 mm too. The median sternal incision was taken and extended down to the navel to dissociate the adhesion between apex and capsular bag. The ascending aorta and right atrium cannula were used to establish cardiopulmonary bypass (CPB), and the PDA was ligated and cut off. When the rectal temperature was dropped to 25 ℃, clamped the ascending aorta, and perfused HTK myocardial protection solution. Then stopped CPB, pushed the aortic cannula to the innominate artery and blocked it, and started anterograde cerebral perfusion with flow rate of 30 mL/kg body weight. Thoroughly resected the isthmus of the aortic arch and the ductus arteriosus tissue, cut it along the long axis at the proximal bottom of the aortic arch, and performed end-to-side anastomosis with the descending aorta (7/0 prolene), then stopped the cerebral perfusion, withdrew and advanced the aortic cannula to the anastomosed aortic arch, clamped the aorta again, then restarted the CPB. Stopped the circulation when the temperature dropped to 18 ℃, and VSD was repaired with bovine pericardial biological patch with 6/0 prolene, and ASD was sutured directly. Sutured the right atrial incision, re-circulate with CPB and re-warm. The cardioversion is sinus rhythm. Delayed chest closure and skin incision was sutured with silastic patch. Total time of CPB: 177 min; aortic clamp time: 102 min; circulatory arrest time: 43+30 min, anterograde cerebral perfusion time: 34 min. Adrenalin and Treprostinil were given after operation.
Day 17 after birth, the edema of the child was relieved, and the second operation was performed, including diaphragm folding and abdominal muscle repair. Day 21 after birth, the third operation, we performed sternal suture (Figure 1C,1D).
The patient recovered smoothly after the operations and has been discharged from hospital at one-month-old. After 6 and 12 months of follow-up, the patient is in good condition (Figure 4).
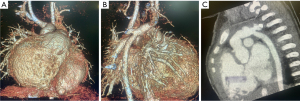
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this case report and accompanying images and video. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
PC is a rare congenital malformation, which was first reported by Cantrell in 1958 and revised by Toyama in 1972 (2). PC can be diagnosed by ultrasound in early pregnancy (3). Prenatal diagnosis helps families to make an informed decision on whether to continue pregnancy. It is extremely rare for twins to have PC (4). In this case, one of the twins has PC, and the other fetus is healthy.
Cantrell’s definition of PC mainly involves the following five aspects, including intracardiac structural malformation, right-lateral heart defect, diaphragmatic pericardium defect, lower sternum defect, defect in front of diaphragm and midline of upper abdomen (1,5). Because it is often complicated with cardiac heterotopia, it is also called thoracic and abdominal cardiac heterotopia. Patients can show the existence of all five defects, or they may only have some defects. It can be divided into complete type and incomplete type (6,7). Defects also vary greatly in degree. For example, the defect of sternum may be manifested as complete loss of sternum, or it may be cracked or shorter than normal. The severity of the disease varies accordingly. In some cases, the heart protrudes beyond the chest and abdomen, as if the heart is beating outside the body, especially in patients with omphalocele and abdominal fissure (3). There are many kinds of deformities in this reported case, which are consistent with the manifestation of complete PC.
Compound malformations are the most common cardiac malformation, especially VSD. ASD, left ventricular diverticulum, pulmonary artery stenosis, tetralogy of Fallot (TOF), and double outlet of right ventricle (DORV) are also frequently reported (8). This child’s cardiovascular malformation is characterized by severe aortic coarctation with arch hypoplasia, which is rarely reported in literature (8).
The general consensus of PC treatment is to make individualized plan according to the complexity of chest and abdominal wall defect and intracardiac malformation. The primary management of newborn babies is particularly important. The strategies include neonatal resuscitation, keeping hemodynamic stability, and possibly need ventilation support, inhaling nitric oxide (NO) and extracorporeal membrane oxygenation (ECMO). Prostaglandins may also be needed to maintain the patency of PDA in order to treat PDA-dependent congenital heart disease. Surgical treatment is an important method for radical treatment of PC. Many methods of reconstruction and repair have been described in the literature. The surgical purposes include correcting cardiac malformation, restoring cardiac position and anatomical structure, and repairing defects of chest and abdomen wall and diaphragm.
The mortality rate of patients with cardiac heterotopia was as high as 52% (9), and the main causes were cardiac rupture, tamponade, cardiac arrest, endocarditis, vascular embolism, heart failure and arrhythmia (3,9). The prognosis of patients with severe complete PC is poor, the average survival time without surgical intervention is about 36 hours (3).
We believe that staged operation is more appropriate, which is consistent with the literature report (7): if the congenital heart disease of PC patients is not so serious as to pose no threat to life, then the early surgical intervention is mainly to repair the abdominal wall muscles and diaphragm, so as to let the heart return to the chest at first, and if the heart structure is abnormal, we can judge whether or when to perform the operation according to the results of long-term follow-up. In recent years, there have been some reports of treatment of some such extraordinary heart defects in PC with transcatheter methods (10,11). However, this reported case was diagnosed with aortic arch hypoplastic and coarctation, and a large VSD. Due to the gradual closure of the PDA, the blood flow in the descending part of the aortic arch is reduced, and the left ventricular pressure is continuously increased, which leads to the rapid occurrence of heart failure. Therefore, it is necessary to complete the correction of cardiac malformation as soon as possible. Similar to conventional heart surgery, correction of cardiac malformation is carried out under CPB. Because of ectopic heart and lack of pericardium in front of the heart, the heart and its blood vessels often adhere to surrounding tissues, so care should be taken not to damage coronary vessels during separating.
Because the child is light in weight, only 2,500 g at the first operation, and there are bilateral superior vena cava, which makes difficulty to cannulate the vena cava, the technique of DHCA was adopted during the operation, and the anterograde cerebral perfusion technique was adopted during the aortic arch reconstruction, thus ensuring the aortic arch forming effect and completely repair of the VSD. Because of postoperative myocardial edema, the strategy of delaying chest closure was adopted, and the abdominal muscles were repaired at the second operation, and the diaphragm was folded, which also created conditions for the heart to completely return to the chest cavity in the next step. Finally, all deformities were corrected and good results were achieved.
The complications of PC range from surgery to anesthesia, and the lack of timely intervention will make further complication of the patient’s condition. In addition, complications of cardiac malformation repair surgery and the postoperative thoracic and abdominal cavity hypertension may lead to cardiac decompensation and intra-abdominal organ damage. Therefore, a multidisciplinary team is needed to manage such patients. The team should consist of obstetricians, neonatologists, radiologists, pediatric cardiologists, pediatric cardiac surgeons and general surgeons (12), thereby reducing the occurrence of complications to the greatest extent and improving the survival rate of patients. The successful treatment of this rare patient with PC demonstrates the experience and teamwork ability of pediatric surgeons. A long-term follow-up is still needed to observe complications such as neurological development in this child.
Conclusions
Cantrell pentalogy is a rare congenital dysplasia, and its condition is complex. Surgery is an effective treatment method, and the prognosis is related to the complexity of the malformations.
Acknowledgments
Funding: This study was supported by Key Laboratory of Structural Deformities in Children of Suzhou, SZS 2022018.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-14/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-14/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-14/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this case report and accompanying images and video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cantrell JR, Haller JA, Ravitch MM. A syndrome of congenital defects involving the abdominal wall, sternum, diaphragm, pericardium, and heart. Surg Gynecol Obstet 1958;107:602-14.
- Toyama WM. Combined congenital defects of the anterior abdominal wall, sternum, diaphragm, pericardium, and heart: a case report and review of the syndrome. Pediatrics 1972;50:778-92.
- Taee N, Goodarzi MF, Safdari M, et al. Pentalogy of Cantrell in Full Term Neonate. AJP Rep 2019;9:e144-6. [Crossref] [PubMed]
- M Demir M. Sertel E, Ture MZ. Twin pregnancy in which both fetuses have Cantrell's pentalogy: A case report and literature review. Eur J Obstet Gynecol Reprod Biol 2021;260:64-9. [Crossref] [PubMed]
- Nayak S, Dash S P, Khatua M. Fetal anomaly: Pentalogy of Cantrell. IOSR J Dent Med Sci 2015;14:52-55.
- Grigore M, Micu R, Matasariu R, et al. Cantrell syndrome in the first trimester of pregnancy: imagistic findings and literature review. Med Ultrason 2020;22:189-96. [Crossref] [PubMed]
- Kylat RI. Complete and Incomplete Pentalogy of Cantrell. Children (Basel) 2019;6:109. [Crossref] [PubMed]
- Jnah AJ, Newberry DM, England A. Pentalogy of Cantrell: Case Report With Review of the Literature. Adv Neonatal Care 2015;15:261-8. [Crossref] [PubMed]
- Zhang XQ, Zhang W, Xiao MD, et al. Surgical diagnosis and treatment of eight cases with pentalogy of cantrell. Chinese Journal of Cardiovascular Review 2011;9:335-9.
- McMahon CJ, Walsh KP. Transcatheter right ventricular outflow tract stent implantation in a child with pentalogy of Cantrell, double outlet right ventricle, and severe pulmonary stenosis. Catheter Cardiovasc Interv 2013;82:1164-7. [Crossref] [PubMed]
- Galeczka M, Fiszer R, Knop MT, et al. Successful atrial septal defect transcatheter closure in a patient with pentalogy of Cantrell and ectopia cordis. Postepy Kardiol Interwencyjnej 2019;15:247-50. [Crossref] [PubMed]
- Rizwan M, Kumar KR, Dass C, et al. Perioperative management of a neonate with Cantrell's pentalogy. Indian J Anaesth 2018;62:827-9. [Crossref] [PubMed]
Cite this article as: Liao J, Huang H, Li X. Surgical treatment of neonatal Cantrell pentalogy: a case report and literature review. AME Case Rep 2023;7:22.


