
The demise of ORISE, a submucosal lifting agent for endoscopic resection
Endoscopic resection of large nonpedunculated colorectal polyps (LNPCPs) results in less morbidity and mortality and lower costs compared to surgical resection (1), and is now preferred over surgical resection for benign LNPCP regardless of their size, shape, or location within the colorectum. While gastroenterology society recommendations advocate endoscopic resection of all benign LNPCPs (2), a reduction in rates of surgical resection for benign polyps has only recently been documented (3). Thus the U.S. has far to go to optimize the safety and cost effectiveness of resection of LNPCP, but an important trend in the best direction appears to be underway.
Endoscopic resection of LNPCP is mostly performed by the techniques of endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) (2). Conventional EMR is performed by injection of fluid into the submucosa below the polyp followed by snare resection. The submucosal fluid cushion separates the LNPCP from the muscularis propria, which is useful for protecting the muscular propria from injury during snare transection. Injury to the muscularis propria can result in immediate or delayed perforation, the most feared complication of endoscopic resection. Another method of EMR called underwater EMR does not involve any submucosal injection, but rather relies on water filling of the lumen to achieve separation of the LNPCP from the muscularis propria (4). This removal of luminal gas and water filling of the lumen causes the mucosa to “float” up and away from the muscle wall, removing any need to separate the LNPCP from the muscularis propria by submucosal injection. ESD involves dissection of the submucosa under the LNPCP using an endoscopic knife, and essentially always includes submucosal injection. Thus, both conventional EMR and ESD rely fundamentally on submucosal injection.
Most endoscopic resectionists include a contrast agent in the submucosal injection fluid, typically blue in color (Table 1). In non-Food and Drug Administration (FDA) approved or “homemade” solutions the contrast is usually indigo carmine or methylene blue, and methylene blue has been used in several FDA approved solutions (Table 1). The blue contrast delineates the lesion border, which can be particularly useful in large sessile serrated lesions, which are notorious for their indistinct margins. More importantly, the contrast stains the submucosa but not the muscularis propria, so muscularis propria exposure or injury during snare resection becomes obvious during EMR (5). During ESD the contrast accentuates the submucosa and helps direct a safe cutting plane away from the muscularis propria.
Table 1
| Compound and company | Active ingredient | Contrast agent | FDA approved date |
|---|---|---|---|
| SIC-8000 (Eleview) (Cosmo Pharmaceuticals) | Poloxamer 188 | Methylene blue | September 2015 |
| Polyoxyl-15-hydroxystearate | |||
| ORISE gel (Boston Scientific) | Gellan gum | FD&C blue No. 1 | September 2018 |
| Polysaccharide | |||
| BlueBoost (Micro-Tech Endoscopy) | Sodium hyaluronate | Methylene blue | June 2021 |
| EverLift (Laborie Medical Technologies) | Cellulose | Methylene blue | July 2020 |
| EndoClot SIS (Olympus) | Absorbable starch polymers | No contrast added | April 2020 |
| LiftUp (Ovesco) | Poloxamers (thermo-sensitive gel formulation) | Methylene blue | August 2022 |
FDA, Food and Drug Administration.
Endoscopists were using “homemade” submucosal injection fluid with contrast added for decades before the FDA approved any submucosal injection fluid. Saline was the prototype fluid and is still preferred by some endoscopists. However, saline has a tendency to dissipate quickly, often spreading laterally through the submucosa with the result of flattening the submucosal cushion under the lesion. Endoscopists explored more viscous solutions, including hydroxypropyl methylcellulose, glycerol, sodium hyaluronidate, and 50% dextrose, among others. Fifty percent dextrose was abandoned because it could induce a painful inflammatory response if inadvertently injected into the pericolonic mesentery (6). The first more viscous injectate tested in a randomized blinded clinical trial was succinylated gelatin, which was found superior to saline on the basis of less volume needed to maintain a submucosal cushion, and lesions were removed in fewer pieces (7). Succinylated gelatin is not available in the U.S., but it shared performance features with hydroxyethyl starch, which became popular in the U.S. as a viscous alternative that is widely available and inexpensive.
In 2015, the compound SIC-8000 became the first submucosal injection agent approved by the FDA. In a blinded randomized trial, using generally the same endpoints used in the succinylated gelatin trial, SIC-8000 outperformed saline as a submucosal injection agent (8). A post-marketing investigator-initiated trial showed SIC-8000 was also superior to hetastarch (9).
Importantly, the FDA approved SIC-8000 not as a drug but as a Class II medical device. This opened the door for other companies to seek FDA approval for new injection fluids based on SIC-8000 as a predicate device, and using largely animal data to establish efficacy and safety (Table 1).
After SIC-8000, the next agent to receive FDA approval was ORISE gel (Boston Scientific, Marlborough, MA, USA) (Table 1). Again, there were few clinical data on ORISE gel at the time of FDA approval, and ORISE was approved as a device based on evidence that it was largely equivalent to SIC-8000. Anecdotally, ORISE seemed to users to have certain differences from SIC-8000. The color of ORISE was a darker blue, which was appreciated during EMR. During ESD, ORISE seemed to produce few bubbles. Bubbles are a feature of SIC-8000 and perhaps other fluids containing an emulsifier. Endoscopists performing ESD find bubbles annoying. As a downside for ORISE, it was not clear that epinephrine could be added to ORISE without affecting it gelling properties. This was a disadvantage since some practitioners prefer to include epinephrine to reduce immediate bleeding. All of these differences seemed typical of slight variations between otherwise fairly equivalent products.
Unfortunately, soon after the launch of ORISE, performance features of ORISE began to be discovered that constituted potentially clinically significant adverse features of the material as a submucosal injection agent. First, the ORISE material was often visible in the submucosa of the resected specimen when the tissue was examined in the pathology department. ORISE in the resected specimen had a basophilic appearance resembling mucin in hematoxylin and eosin stained tissue, though mucin stains were negative (10-12). This issue seemed easily overcome by warning clinical pathologists that ORISE had been used. However, ORISE appeared to persist in the tissue, and biopsies from specimens taken at a later date showed an eosinophilic material accompanied by a multi-nucleated giant cell reaction (12-17).
Patients undergoing piecemeal EMR typically have a follow-up examination 6–12 months after EMR to verify complete resection (2). In my EMR practice, we began noticing that some EMR sites after ORISE injection demonstrated submucosal fullness and distortion when viewed 6–12 months after EMR. In one instance, a “bite-on-bite” biopsy technique led to a pasty yellow-orange material exuding through the bite site and deflating the submucosal distortion (18). I did not consider this submucosal distortion to interfere with the interpretation of the EMR scar, or to interfere with resection of any residual polyp. Further, this distortion was easily differentiated from “clip artifact”, which is a nodular mucosal distortion resulting from clip placement to close EMR defects at the time of the original resection (19). Thus, it seemed this submucosal distortion at follow-up could be another ORISE consequence without major clinical implications. However, during a monthly live web-based course on EMR that was broadcasted from my center, a physician asked if I had seen distorted EMR scars after ORISE. When I replied “yes” he related a story from his home institution in which submucosal distortion was seen at follow-up of a rectal EMR scar after an EMR that employed ORISE injection. The distortion led to concern of submucosal tumor growth, which was followed by surgical resection. However, there was no neoplasia in the resected specimen, only a submucosal collection of eosinophilic material and multi-nucleated giant cells consistent with an ORISE reaction. These changes occurring in response to ORISE have been termed “lifting granuloma” (13), and this story meant that submucosal distortion in an EMR scar could lead to an important adverse clinical outcome.
In response my research team organized a blinded expert review of endoscopic photographs of 30 ORISE EMR scars and 30 EMR scars after injection of other fluids, which confirmed that the tissue under ORISE scars was often distorted (18). In response to reports of this type, Boston Scientific first issued a warning to users, and then on December 15, 2022, Boston removed ORISE from the market worldwide. Figure 1 shows an example of submucosal mass effect and deformity seen at follow-up colonoscopy 6 months after EMR using ORISE.
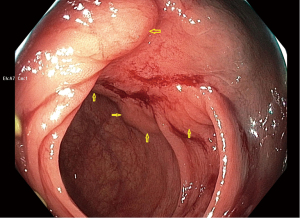
In a current case report published on AME Case Reports, Mendelson et al. describe 3 cases of surgical resection of colorectal EMR of sites when ORISE had been used (20). In all 3 cases there was a mass effect related to the injected material that caused diagnostic confusion, and sometimes technical difficulty performing a surgical resection. In case 3 ORISE related distortion was the likely cause of endoscopist concern for invasive cancer under an EMR site, which lead to an unnecessary surgical resection as there was no residual neoplasia in the surgical specimen. The mass effect and histological changes in these cases involved the submucosa, muscularis propria, and subserosa including subserosal blood vessels. Another very recent case series reports that ORISE injection commonly produces a persistent mass effect that can be present in the submucosal or in all layers of the bowel wall and which could create clinical diagnostic confusion as to whether an intramural neoplasm is present (21). These cases support Boston Scientific’s responsible decision to remove ORISE from the market.
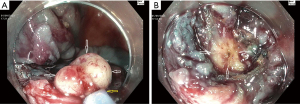
Table 2 lists the adverse clinical effects of ORISE gel use, several of which are discussed above. Another adverse clinical effect not yet described in the ORISE publications, but which we have encountered on several occasions, occurs when a referring physician tests whether a colorectal lesion lifts well (Table 2). This test is typically performed by a referring physician when the lesion is first detected, and is one way to check the resectability of the lesion, as failure to lift can suggest invasive cancer. Our experience is that if this test is performed with ORISE, and the patient is then referred to our center, we encounter a lesion with submucosal distortion and mass effect of the type we have described above, but of course the lesion is not yet resected. The lesion then fails to lift with further injection because the submucosa in stiff from the ORISE gel. Snare transection is tough and the foreign material is evident in the submucosa (Figure 2). Histology demonstrates the foreign material and a multi-nucleated giant cell reaction. Like the other effects of ORISE gel, this tough submucosa raises concern for cancer when first encountered.
Table 2
| Effect of ORISE | Consequence |
|---|---|
| ORISE visible in resection specimen | Pathologist mistakes material for mucin or amyloid |
| ORISE injected to test lifting before referral to resectionist | Submucosa tough at time of endoscopic resection; foreign material interferes with further lifting; mimics submucosal invasion during transection |
| ORISE used as endoscopic mucosal resection lifting agent leading to mass effect in submucosa and muscularis propria that is visible at endoscopic follow-up or during surgical resection | Endoscopist believes mass effect is tumor in wall and refers patient to surgery |
| Surgeon sees mass effect and believes cancer present | |
| Mass effect interferes with resection, anastomosis creation |
Several questions remain about ORISE. Which component of the injectate persisted in the tissue and which induced the foreign body reaction? The likely culprit is the gellan gum, a food additive with gelling properties that had not been previously used as a submucosal lifting agent. Did injection technique affect whether the reaction occurred? Most importantly, is the device pathway chosen by the FDA the best approach to injection agents? Did the FDA perform an adequate investigation of ORISE before approving the material for use in humans? Unlike ORISE, several of the FDA approved agents are based on viscous materials with an established safety record in clinical studies before FDA approval (Table 1). Thus, we may have seen the end of adverse clinical outcomes caused by the type of clinical confusion and incorrect diagnoses induced by ORISE gel. Only time will tell.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Case Reports. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-15/coif). DKR reports that consulting fees was supported by Olympus Corporation, Boston Scientific, Braintree Laboratories Norgine, Endokey, GI Supply, Medtronic, *Acacia Pharmaceuticals (*not active in the last 12 months) and the other financial or non-financial interests was supported by Olympus Corporation, Medivators, Erbe USA Inc., Braintree Laboratories Shareholder-Satisfai Health. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jayanna M, Burgess NG, Singh R, et al. Cost Analysis of Endoscopic Mucosal Resection vs Surgery for Large Laterally Spreading Colorectal Lesions. Clin Gastroenterol Hepatol 2016;14:271-8.e1-2.
- Kaltenbach T, Anderson JC, Burke CA, et al. Endoscopic Removal of Colorectal Lesions-Recommendations by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020;158:1095-129. [Crossref] [PubMed]
- Kruger AJ, Hussan H, Stanich PP, et al. Postoperative Hospital Outcomes of Elective Surgery for Nonmalignant Colorectal Polyps: Does the Burden Justify the Indication? Am J Gastroenterol 2021;116:1938-45. [Crossref] [PubMed]
- Kamal F, Khan MA, Lee-Smith W, et al. Underwater vs conventional endoscopic mucosal resection in the management of colorectal polyps: a systematic review and meta-analysis. Endosc Int Open 2020;8:E1264-72. [Crossref] [PubMed]
- Holt BA, Jayasekeran V, Sonson R, et al. Topical submucosal chromoendoscopy defines the level of resection in colonic EMR and may improve procedural safety (with video). Gastrointest Endosc 2013;77:949-53. [Crossref] [PubMed]
- Katsinelos P, Kountouras J, Paroutoglou G, et al. A comparative study of 50% dextrose and normal saline solution on their ability to create submucosal fluid cushions for endoscopic resection of sessile rectosigmoid polyps. Gastrointest Endosc 2008;68:692-8. [Crossref] [PubMed]
- Moss A, Bourke MJ, Metz AJ. A randomized, double-blind trial of succinylated gelatin submucosal injection for endoscopic resection of large sessile polyps of the colon. Am J Gastroenterol 2010;105:2375-82. [Crossref] [PubMed]
- Repici A, Wallace M, Sharma P, et al. A novel submucosal injection solution for endoscopic resection of large colorectal lesions: a randomized, double-blind trial. Gastrointest Endosc 2018;88:527-35.e5. [Crossref] [PubMed]
- Rex DK, Broadley HM, Garcia JR, et al. SIC-8000 versus hetastarch as a submucosal injection fluid for EMR: a randomized controlled trial. Gastrointest Endosc 2019;90:807-12. [Crossref] [PubMed]
- Cypher L, Sun S, Forster E, et al. Submucosal lifting agent ORISE gel remnants histopathologically mimic mucin and malignancy: a case series. Am J Clin Pathol 2019;152:S73. [Crossref]
- Esnakula AK, Liu X, Draganov PV, et al. ORISE Gel: A Submucosal Lifting Agent Mimics Mucin in Endoscopic Resection Specimen. ACG Case Rep J 2020;7:e00403. [Crossref] [PubMed]
- Dong ZM, Fang J, Byrne KR, et al. Histologic mimics and diagnostic pitfalls of gastrointestinal endoscopic lifting media, ORISE™ gel and Eleview®. Hum Pathol 2022;119:28-40. [Crossref] [PubMed]
- Westbrook LM, Henn PA, Cornish TC. Lifting Agent Granuloma: Histologic Findings Following Use of ORISE Gel for Endoscopic Resections in the Gastrointestinal Tract. Am J Clin Pathol 2020;153:630-8. [Crossref] [PubMed]
- Pezhouh MK, Burgart LJ, Chiu K, et al. Characterization of Novel Injectable Lifting Agents Used in Colonic Polyp Removal: An Emerging Amyloid Mimic. Am J Surg Pathol 2020;44:793-8. [Crossref] [PubMed]
- Olivas AD, Setia N, Weber CR, et al. Histologic changes caused by injection of a novel submucosal lifting agent for endoscopic resection in GI lesions. Gastrointest Endosc 2021;93:470-6. [Crossref] [PubMed]
- Sun BL. Submucosal lifting agent ORISE gel causes extensive foreign body granuloma post endoscopic resection. Int J Colorectal Dis 2021;36:419-22. [Crossref] [PubMed]
- Castrodad-Rodríguez CA, Panarelli NC, Gersten AJ, et al. Features of endoscopic procedure site reaction associated with a recently approved submucosal lifting agent. Mod Pathol 2020;33:1581-8. [Crossref] [PubMed]
- Lahr RE, DeWitt JM, Zhang D, et al. Assessment of submucosal distortion and mass effect seen at follow-up after colorectal EMR with ORISE (with video). Gastrointest Endosc 2022;96:679-82. [Crossref] [PubMed]
- Sreepati G, Vemulapalli KC, Rex DK. Clip artifact after closure of large colorectal EMR sites: incidence and recognition. Gastrointest Endosc 2015;82:344-9. [Crossref] [PubMed]
- Mendelson NL, Elliott KR, Evans KE, et al. Lifting agent granuloma presenting as a colonic mass mimicking cancer: a report of three cases. AME Case Rep 2023;7:6. [Crossref] [PubMed]
- Colak Y, Hasan B, Tandon K, et al. Potential clinical complications of Orise™ gel use, a new submucosal lifting agent: experience from a tertiary care center and review of the literature. Ann Gastroenterol 2022;35:407-13. [PubMed]
Cite this article as: Rex DK, Lahr RE. The demise of ORISE, a submucosal lifting agent for endoscopic resection. AME Case Rep 2023;7:11.


