
Post-polio syndrome presenting as isolated neck extensor myopathy: a case report
Highlight box
Key findings
• Isolated neck extensor myopathy presenting as a part of the post-polio syndrome is a likely but rarely documented occurrence.
What is known and what is new?
• Following recovery from polio, survivors may experience a constellation of symptoms caused by prolonged stress on musculoskeletal structures and increased metabolic demand on surviving motor neurons.
• This case demonstrates that truncal hypertonia can occur, but it is not always recognized.
What is the implication, and what should change now?
• A multimodal intervention focused on core muscle training, cervical and lumbar manipulation, and avoiding overuse of the weak muscles significantly improved neuromuscular symptoms, global posture, and spinal function.
Introduction
Poliomyelitis, or polio, is a contagious disease caused by poliovirus. Approximately 70% of polio infections are asymptomatic (1). Symptomatic cases can be divided into three clinical manifestations: abortive polio (non-specific minor illness), non-paralytic polio (aseptic meningitis without paralysis), and paralytic polio (flaccid paralysis of various muscle groups). Paralytic polio occurs in less than 1% of all infections (1,2). The disease, depending on the level of involvement, is classified as spinal polio (spinal cord), bulbar polio (brainstem), or bulbospinal polio (both). Spinal polio accounts for 79% of paralytic cases and is characterized by asymmetric paralysis that most often involves the legs. The paralysis is usually permanent, but some recovery may occur through compensation from the unaffected neighboring nerve cells and muscles (1).
Polio survivors may experience a constellation of symptoms post-recovery, called post-polio syndrome (PPS) (2). PPS was first coined by Halstead in 1986, who presented criteria for the diagnosis of PPS, including new or deterioration of muscular weakness and atrophy, fatigability, muscular and joint pain, and cold intolerance several years after acute paralytic polio infection (3). All these latent sequelae of polio are the result of the damage incurred by initial infection, prolonged stress on musculoskeletal structures, and increased metabolic demand of surviving motor-neurons (4,5). Head ptosis (head drop or dropped head syndrome) is a rare condition due to the weakening of neck extensors to hold the head in an upright position. Isolated neck extensor myopathy (INEM) is a specific type of head ptosis caused by non-inflammatory myopathy restricted to the cervical extensor muscles (6).
Wild poliovirus has been eradicated in the developed countries by the introduction of the Salk (in 1955) and Sabin (in 1962) inactivated poliovirus vaccines (1). As of the year 2022, endemic circulation of wild poliovirus persists only in Afghanistan, Pakistan, and poor African countries where vaccination is difficult (7). About 15% to 80% of the polio survivors will get a deterioration of symptoms, effective treatment for the neuromuscular sequelae, especially core muscle weakening, in the 20 million global patients of paralytic polio is desperately needed (8). There is a paucity of data describing or reporting on the outcomes of conservative treatment for PPS and head ptosis. The current study highlights the potential effectiveness of a chiropractic approach in addressing head ptosis and posture problems in a patient at a post-polio stage. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-76/rc).
Case presentation
A 56-year-old Asian businessman reported that he got paralytic poliomyelitis when he was 5 years old, which subsequently resulted in right lower limb flaccid paralysis. The patient had made a partial recovery and kept his neuromuscular functioning largely steady for the next five decades after the infection. He has been able to walk ever since with the aid of a cane and a foot orthotic. However, he started experiencing deep ache in the nuchal and low back regions, and general fatigue one year before first seek for chiropractic care. The patient denied any previous injuries. His primary care physician radiographically diagnosed him with degenerative cervical and lumbar spondylosis at the time. Tricyclic antidepressants, clonazepam, and tramadol were prescribed for pain relief on an as-needed basis. Despite taking the aforementioned medications, the neck and back pain and fatigue got worse, and the difficulty to hold the head up progressed over the course of the following six months. The spinal pain and head ptosis had severely limited his activities of daily living more than before, and forced him to seek further treatment.
The patient presented with a dropped head posture and was barely able to hold his head up for 5 minutes. The head ptosis was correctable in the supine position. He ambulated with a cane with a short step length and was accompanied by a foot drop on the right side. In addition, the patient had atrophy on the right leg. Palpation revealed hypertonicity of the bilateral upper trapezius, rhomboids, and quadratus lumborum muscles, and intersegmental motion restrictions were found from C5–7, T3–8, and L2–5. The intensity of the neck and low back pain was rated at 5–6/10 on a 1–10 numeric pain rating scale, where 0 means no pain and 10 means the worst imaginable pain. His range of motion in the cervical region was recorded at 90° of flexion, 30° of extension (normal 50°–70°), 10° of lateral flexion (normal 20°–45°), and 90° of rotation to both sides.
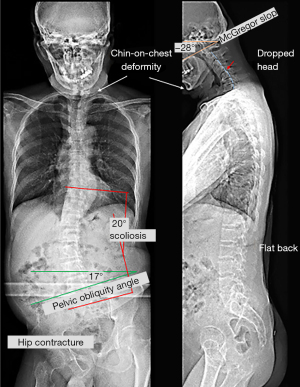
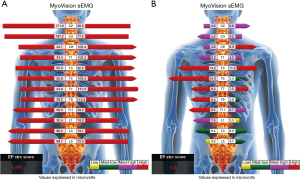
The manual strength grade of the right lower limb was 2/5. The deep tendon reflex test, using a reflex hammer, was conducted by a chiropractor, showing hyporeflexia of the right knee-jerk and ankle-jerk. The neurological findings of the left leg were normal. Standing full-spine EOS® radiographs showed reverse cervical lordosis with anteroflexion deformity, osteophytes in the lower cervical and lumbar spine, and interspace narrowing of the C3/C4, C5/6, C6/C7, C7/T1, and L4/5 levels, suggestive of degenerative spondylosis. A coronal view revealed right pelvic drop, right hip contracture with abduction, and a long thoracolumbar curve (T10–L5 Cobb angle of 20°) with a right convexity as compensatory changes for pelvic obliquity (Figure 1). The World Health Organization Quality of Life (WHOQOL-OLD) (9) scoring was used, and the results were linearly translated to a 0–100 scale, with higher scores indicating better quality of life. The patient received a score of 48. Static surface electromyography (sEMG) was conducted using MyoVision® 4000 (Precision Biometrics/MyoVision, San Carlos, CA, USA) revealed intense myoelectric activity of bilateral paraspinal muscles throughout the spine (Figure 2). This apparatus has been shown to be reliable among both healthy and neck-pain individuals (10). The patient met the diagnostic criteria for PPS (2). PPS related isolated neck extensor myopathy (INEM) was impressed.
The initial phase of chiropractic care was aimed at relieving pain and improving core muscle functions. During each visit, the intervention included: (I) 15-minute mechanical cervical traction to reduce the pressure on the nerves; (II) 8-minute cervical and lumbar manual therapy to improve mobility; (III) 8-minute deep muscle massage using a handheld micro-vibrator (Strig®, STRIG Inc., Korea) to relax the hypertonic muscles; and (IV) 15-minute isometric core trunk muscle training (11) to strengthen the paraspinal muscles and improve spine posture. The patient was seated in a commercially robotic chair (AllCore360° Core Training System®, AllCore360, GA, USA) for the workout. The device is designed to allow for increasing the strength of the core muscles by progressively contracting the trunk muscles while the chair slowly rotates at various angles and the patient keeps his spine in a neutral position (Figure 3).

Initial treatment sessions were scheduled 3 times a week for the first month, focusing on pain symptoms. Then, the frequency of treatment was reduced to 2 times a week for 2 months, focusing on relieving pain and improving core muscle functions. Afterwards, the patient was seen once a week for 10 months, focusing on correcting both pelvic and spinal postures. The frequency of treatment was further reduced to once a month for 30 months, focusing on monitoring the symptoms, spinal adjustment, and core muscle training. A home stretching program (muscle strengthening, mobility training, and ergonomic recommendations) was also used as an adjunct to care. When training with exercise programs, the standard advice is to avoid exercising too hard and to train at submaximal but progressively increasing levels.
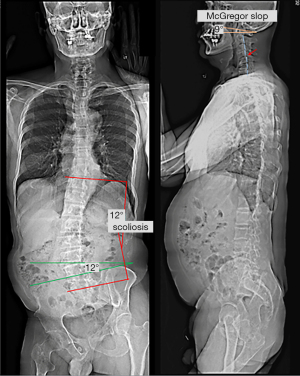
The patient reported that his numeric pain scale was reduced from 6 to 2 on the 1–10 scale after a few visits. His neck mobility was nearly normal and his pain and muscle weakness were in complete remission after four weeks of treatment. His WHOQOL score increased from 48% to 78% at 11 months, indicating improvement in the quality of life. Treatment was continued until either the patient’s improvement plateaued or the maximal improvement was achieved. Forty months after the treatment initiation, follow-up radiographs and sEMG were taken. Comparing to pre-intervention sEMG, paraspinal myoelectric activity returned to normal after intervention at the majority of spinal levels (Figure 2). Repeated radiographs revealed improved head posture, relatively restored normal pelvic obliquity, and lumbar scoliosis reduced to a T10–L5 Cobb angle of 16° (Figure 4). No pathological or treatment-related adverse effects were observed.
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Acute polio is no longer a persistent threat to people in the polio-free areas of the world, but there are an estimated 20 million polio survivors with PPS globally (12,13). The etiology and pathogenesis of PPS are unknown. Survivors frequently recover only partially from their paralysis. It has been proposed that a certain amount of recovery is due to the ability of unaffected neighboring nerve cells to “sprout” and reconnect to the paralyzed muscles. The gradual and latent deterioration of muscle weakness is related to years of overuse of the compensatory motor units that can no longer respond properly, unveiling the neurological impairment caused by the initial illness (13,14). The European Federation of Neurological Societies (EFNS) criteria for the diagnosis of PPS (2,3) are as follows: (I) previous episode of paralytic polio; (II) rehabilitation followed by over 15 years of steady neuromuscular function; (III) new or worsening muscle weakness; (IV) symptoms last at least a year; (V) exclusion of other possible causes of symptoms. Common symptoms include muscle weakness, fatigability, and muscle and joint pain, which are hypothesized to be caused by muscle overuse, leg length discrepancy, asymmetric biomechanics, asymmetric loading on the axial spine, and age-related changes (12). PPS symptoms and functional impairments can have an influence on quality of life and capacity to accomplish everyday duties.
Head ptosis is a rare disorder characterized by the inability to extend the neck due to the weakening of the neck extensor muscles, resulting in a chin-on-chest deformity (6,15). It can be painful, disfiguring cosmetically, impeding swallowing and impairing forward gaze. The pathophysiology of ptosis of the head remains unclear. It might be the presenting symptoms of a vast array of metabolic, dystrophic, myopathic, or neurological disorders (16). INEM is one of the probable causes of head ptosis due to isolated myopathic changes resulting from chronic injury and overloading of the cervical extensor muscles (6). There is a paucity of documented cases reporting a link between head ptosis and PPS. Our interpretation of this association is that polio left our patient with right leg weakness, a length discrepancy, and pelvic obliquity, which led to asymmetric axial loading, scoliosis, and imbalanced core muscles. Overuse and fatigue of the cervical paraspinal muscles (neck extensors) contributed to the inability of the patient to maintain an erect head position. In addition, the scoliotic curvature exerts an additional asymmetric strain on the neck muscles, resulting in INEM. Initial sEMG findings of intense myoelectric activity were consistent with the significant effort require by the cervical paraspinal muscles to prevent the head from dropping onto the chest. A decrease in lumbar myoelectric activity was noted when compared with sEMG before and 40 months after intervention (Figure 2). This was attributed to the patient’s asymmetric quadratus lumborum paralysis following the initial polio infection.
There are currently no established regimens that can prevent the deterioration of PPS. Generally, the overall goal of the management of PPS is symptomatic, aiming to reduce pain and improve function by minimizing the excessive metabolic stress on the muscles (17). Evidence suggests that in order to treat new weakness in patients with PPS, supervised training programs are required (18). These programs focus on boosting muscle strength through non-fatiguing exercises that involve using submaximal or maximal strength with short-duration repetitions on alternate days (2,18). Such program yielded favorable results, by facilitating a full recovery from the exercises within the constraints of fatigue and pain (19) and preventing the overuse of the weak muscle (17). On follow-up sEMG (Figure 2) and radiographs (Figure 4), relaxation of paraspinal muscle spasm, improvement of spinal alignment, and restoration of normal pelvic obliquity may have contributed to enhanced spinal functional capacity of the current case. This case illustrates that hypertonic muscle may be present, a fact that is not always acknowledged.
This study has potential limitations. First, due to the rarity of both PPS and INEM, this was a single case study and was not controlled. Second, PPS was basically diagnosed on a medical history and the exclusion of other conditions that might explain the symptoms. Electromyography and magnetic resonance imaging for a thorough investigation of soft tissue alteration in the neck had not been taken. Furthermore, the precise effectiveness of chiropractic intervention could not be clarified by the multimodal treatment strategy. The mechanisms of symptom relief and posture retrieval warrant further scrutiny. The strength of this work is that it provides information indicating that the occurrence of INEM decades after paralytic polio infection is a probable but rarely documented event.
Conclusions
The current study was the first to report INEM among patients with PPS. The suggested multimodal intervention with long-term spinal manual therapy has shown significant improvement in neuromuscular symptoms, the head and global posture, and spinal function.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-76/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-76/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Estivariz CF, Link-Gelles R, Shimabukuro T. Chapter 18: Poliomyelitis. In: Hamborsky J, Kroger A, Wolfe C, eds. Epidemiology and Prevention of Vaccine-Preventable. 13th ed., Washington, DC: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2020:297-310.
- Farbu E, Gilhus NE, Barnes MP, et al. EFNS guideline on diagnosis and management of post-polio syndrome. Report of an EFNS task force. Eur J Neurol 2006;13:795-801. [Crossref] [PubMed]
- Halstead LS. Assessment and differential diagnosis for post-polio syndrome. Orthopedics 1991;14:1209-17. [Crossref] [PubMed]
- Godzik J, Lenke LG, Holekamp T, et al. Complications and outcomes of complex spine reconstructions in poliomyelitis-associated spinal deformities: a single-institution experience. Spine (Phila Pa 1976) 2014;39:1211-6. [Crossref] [PubMed]
- Chu ECP, Lam KKW. Post-poliomyelitis syndrome. Int Med Case Rep J 2019;12:261-4. [Crossref] [PubMed]
- Chu EC, Wong AY, Lin AF. Isolated Neck Extensor Myopathy Associated With Cervical Spondylosis: A Case Report and Brief Review. Clin Med Insights Arthritis Musculoskelet Disord 2020;13:1179544120977844. [Crossref] [PubMed]
- Shabbir H, Saeed S, Farhan M, et al. Poliomyelitis in Pakistan: Challenges to polio eradication and future prospects. Ann Med Surg (Lond) 2022;80:104274. [Crossref] [PubMed]
- Oluwasanmi OJ, Mckenzie DA, Adewole IO, et al. Postpolio Syndrome: A Review of Lived Experiences of Patients. Int J Appl Basic Med Res 2019;9:129-34. [Crossref] [PubMed]
- Liu R, Wu S, Hao Y, et al. The Chinese version of the world health organization quality of life instrument-older adults module (WHOQOL-OLD): psychometric evaluation. Health Qual Life Outcomes 2013;11:156. [Crossref] [PubMed]
- Kima GE, Yunb DU, Anb YJ, et al. Reliability and validity of new evaluation methods using static surface electromyography in persons with neck pain. Phys Ther Rehabil Sci 2019;8:1-7. [Crossref]
- Palevo G, Walsh D, Park E, et al. Physiological responses to Allcore360° Core Training System. Journal of Exercise Physiology Online 2021;24:67-73.
- Farbu E. Update on current and emerging treatment options for post-polio syndrome. Ther Clin Risk Manag 2010;6:307-13. [Crossref] [PubMed]
- Mafla-Ayub KA, Guzmán-Molano LF, Centanaro-Meza GA, et al. The legacy of polio: 2 cases of post-polio syndrome and review. Rev Mex Neurocienc 2022;23:97-104. [Crossref]
- Dalakas MC. The post-polio syndrome as an evolved clinical entity. Definition and clinical description. Ann N Y Acad Sci 1995;753:68-80. [Crossref] [PubMed]
- Petheram TG, Hourigan PG, Emran IM, et al. Dropped head syndrome: a case series and literature review. Spine (Phila Pa 1976) 2008;33:47-51. [Crossref] [PubMed]
- Kesserwani H. Predominant Neck Extensor Muscle Weakness: A Rare Manifestation of Idiopathic Polymyositis. Cureus 2020;12:e8735. [Crossref] [PubMed]
- Jubelt B, Agre JC. Characteristics and management of postpolio syndrome. JAMA 2000;284:412-4. [Crossref] [PubMed]
- Koopman FS, Beelen A, Gilhus NE, et al. Treatment for postpolio syndrome. Cochrane Database Syst Rev 2015;CD007818. [PubMed]
- Agre JC, Rodriquez AA. Muscular function in late polio and the role of exercise in post-polio patients. NeuroRehabilitation 1997;8:107-18. [Crossref] [PubMed]
Cite this article as: Chu ECP, Zoubi FA. Post-polio syndrome presenting as isolated neck extensor myopathy: a case report. AME Case Rep 2023;7:18.


