
Primary central nervous system lymphoma presenting as an isolated intramedullary spinal cord lesion: a case report
Introduction
Despite itself being a relatively rare entity, intramedullary spinal cord tumours (IMSCT) have a variety of histological origins. More than 80% originate from glial tissue (ependymoma or astrocytoma) (1,2). Less than 1% of IMSCT have a haematological source, such as primary central nervous system (CNS) lymphoma. Similarly, primary CNS lymphoma (PCNSL) presenting in the spine is exceedingly rare (<1%), compared to the more commonly encountered intracranial location (1,3). In this report, we present a patient with sarcoidosis who was diagnosed with PCNSL in the spinal cord which itself had atypical features including non-reactivity to steroid. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-41/rc).
Case presentation
In June 2021, a 64-year-old male presented with a 5-month history of progressive ascending peripheral sensory neuropathy. Specifically, he presented with a loss of touch sensation from level T5-S5 and C7/8-T1. He maintained normal limb power, temperature sensation, pain sensation and proprioception. He reported that although he had lost saddle sensation and bowel urgency, he made a routine to visit the bathroom regularly to avoid incontinence. He was able to pass urine and had no abdominal discomfort. He was still able to walk, albeit with a wide based stance and using a walking stick for support. He was independent with activities of daily living, such as eating with cutlery and using a mobile phone, but would often drop items. He was clumsy with his hands, and holding glasses was very difficult.
Initial imaging performed, magnetic resonance imaging (MRI) brain/whole spine (June 2021) (Figure 1), showed an expanded cervical spinal cord with high signal change and oedema from the craniocervical junction to T1 level. Contrast enhancing intramedullary expansile lesion at the C3-C4 level. There were no other lesions identified within the cord. Scattered bilateral periventricular and deep white matter signal changes, resembling small vessel ischaemic disease, were seen supratentorially.
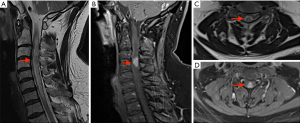
His background included sarcoidosis diagnosed in January 2021 with recurrent granulomatous inflammatory lung disease and associated mediastinal lymph node involvement requiring previous lobectomy. For this, he took oral methotrexate 20 mg weekly for the past 3 months. Prior to methotrexate, he had been on oral prednisone 20 mg daily for 5 months. He had recurrent right thigh necrotising granulomatous panniculitis (biopsy 2019, 2020). He was an ex-smoker with chronic obstructive pulmonary disorder and recurrent multi-drug resistant bacterial pneumonia.
He was initially treated by the medicine and neurology team for neurosarcoidosis based on his previous diagnosis of pulmonary sarcoidosis. Treatment included high dose steroids (intravenous methylprednisolone), cyclophosphamide and infliximab with no clinical benefit. Despite treatment, his symptoms progressed and he began developing limb weakness.
Interval imaging was performed, MRI brain/whole spine (October 2021) (Figure 2), which showed disease progression in the cervical spine with enlargement of the contrast enhancing spinal cord lesion to the mid-point of C5. Intra-cranial new disease progression with enlargement of the left lateral choroid plexus, ependymal enhancement and T2 hyper dense signal changes to posterior thalamus.
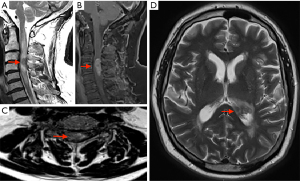
A surgical opinion was then sought. The main priority was to obtain a tissue diagnosis to aid with treatment. The request was for a sample to be taken from the ependymal thickening. Despite the risks of worsening tetraparesis, a biopsy from the cervical spine lesion was thought to provide a greater chance of reliable tissue for histological diagnosis with less bleeding risk than taking a ventricular ependymal tissue biopsy.
In November 2021 the patient underwent C3-4 laminectomy, spinal cord lesion biopsy and duraplasty. On induction for surgery a urinary catheter was inserted and nearly 2,000 mL of urine was drained. Laminectomy of C3 and C4 provided sufficient access for an adequate dural window. The cord generally had good vascularity with a small area where surface vessels were absent as seen in Figure 3. This coincided with the position of the lesion. The dura was noticed to be abnormally thickened when opened. The cord was opened and gentle blunt dissection revealed the lesion which appeared vascular. The lesion did not have an obvious macroscopic margin, but abnormal tissue was easily removed for biopsy.
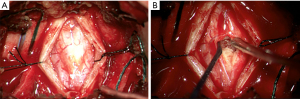
Histology report
Macroscopic: tan red tissue
- Microscopic (Figure 4): glial tissue with infiltrate of large lymphoid cells with irregular nuclei and prominent nucleoli with abundant mature lymphocytes, histiocytes and plasma cells. Perivascular aggregation. Brisk mitotic activity.
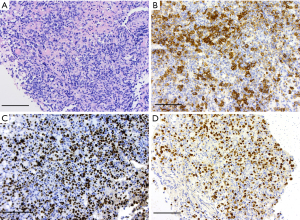 Figure 4 Histology photographs. (A) Hematoxylin and eosin stain: large lymphoid cells. Scale bar: 50 µm. (B) Immunohistochemistry: CD20 marker positivity. Scale bar: 50 µm. (C) Immunohistochemistry: Ki67 proliferation marker. Scale bar: 50 µm. (D) In-situ hybridization: EBER-ISH marker. Scale bar: 50 µm. EBER-ISH, Ebstein-Barr encoded ribonucleic acid in situ hybridization.
Figure 4 Histology photographs. (A) Hematoxylin and eosin stain: large lymphoid cells. Scale bar: 50 µm. (B) Immunohistochemistry: CD20 marker positivity. Scale bar: 50 µm. (C) Immunohistochemistry: Ki67 proliferation marker. Scale bar: 50 µm. (D) In-situ hybridization: EBER-ISH marker. Scale bar: 50 µm. EBER-ISH, Ebstein-Barr encoded ribonucleic acid in situ hybridization. - Immunohistochemistry: large lymphoid cells which were positive for CD20, CD79a, BCL2, MUM1, Ebstein-Barr encoded ribonucleic acid in situ hybridization (EBER-ISH). Negative for CD3, CD10, BCL6, C Myc, broad-spectrum cytokeratin, SOX10, GFAP. Ki67 proliferation index high (90%).
- Conclusion: Epstein-Barr virus (EBV)-positive high grade large B-cell lymphoma.
Given his co-morbidities, he was initially offered palliative radiation therapy (external beam radiation therapy) to the cervical spine for 45 Gy 25 fractions, however only 3.60 Gy 2 fractions were given. It was complicated by enterococcal bacteraemia and meningitis. Interim imaging showed progressive multifocal lymphomatous disease with new nodular enhancing disease in the choroid plexus. Staging CT did not show extra-CNS disease. He did not have a 18F-FDG PET done. An EBV nuclear antigen (EBNA) antibody test was reactive suggesting prior infection by EBV.
He was placed on rituximab and high dose methotrexate (R-MTX). Interval MRI showed improvement in both intracranial and spinal lymphomatous disease. He had a total of 7 cycles. Clinically he remained deconditioned and intermittently confused. Subsequent MRI showed treatment related leukoencephalopathy. He was discharged on maintenance chemotherapy (procarbazine).
At the onset, the oncologists decided that the patient was not for MATRix (methotrexate, cytarabine, thiotepa, rituximab) chemotherapy due to his history of recurrent infections and immunosuppression. Nevertheless, he suffered multiple complications during his prolonged hospital admission including pneumonia, diverticulitis, clostridium difficile gastroenteritis, fungal groin infection, and upper limb deep vein thrombosis (DVT).
Three months post-operation, he was transitioned to inpatient rehabilitation. Unfortunately, he continued to suffer infectious sequelae including urinary tract infection, delirium and focal seizures. With no meaningful progress from physical rehabilitation, the decision was made with the family to progress to Te Ara Whakapiri (Last Days of Life) protocol and he soon passed away in March 2022.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
This case highlights a patient with an intramedullary spinal cord lesion that was eventually diagnosed as a PCNSL.
PCNSL is a rare form of extranodal non-Hodgkin lymphoma. It typically presents in the brain, eyes or leptomeninges (3,4). Risk factors include immunosuppression [acquired immunodeficiency syndrome (AIDS), organ transplant, congenital immune deficiency] and increasing age (5). More than 80% are B cell lymphoma with the most common subtype being diffuse large B-cell lymphoma (DLBCL) (5,6). PCNSL in the spine is rare (3,4). Immunosuppressed patients with PCNSL have different characteristics compared to immunocompetent. For some, the underlying cause is immunosuppressive medications for autoimmune conditions. For example, there is an almost uniform association with EBV in immunosuppressed patients and they also tend to present at an earlier age (less than 65 years old) (7).
PCNSL presents a diagnostic challenge as its presentation and neuroimaging mimics other progressive neurological disorders such as other CNS tumours, demyelinating disease, autoimmune conditions or CNS infection (3). It can easily be misdiagnosed as was the case for this patient. Hachicha found diagnosis took a mean duration of approximately 16 months from symptom onset (2). However, in contrast to other tumours, there was an earlier need for inpatient admission with mean duration from symptom onset being 6 months (8). Our patient was admitted 5 months after symptom onset.
IMSCT can present with variable neurological symptomatology. Typically, it is progressive myelopathy with insidious onset (>8 weeks) (4). Depending on the local mass effect, patients may complain of back pain and a variety of limb neurology such as weakness and paresthesia. Pain (72%) in the back or down the limbs is the predominant symptom. Less commonly, there is motor weakness (55%), sensory loss (39%) and sphincter disturbance (15%) (1,9).
MRI is the preferred imaging modality. Typically, PCNSL in the spine presents as an hyperintense lesion on T2W (in contrast to intracranial lymphoma) (4,10). There is homogenous enhancement on T1W (1,4,5). However, these features are also seen with astrocytoma, metastases and neurosarcoidosis (4). Other features supporting lymphoma include multi-focality (intracranial and leptomeningeal involvement) and peripheral ring-enhancement (3,4,7). Diffusion-weighted imaging (DWI) shows restricted diffusion due to high cell density (1). Fluid-attenuated inversion recovery (FLAIR) may show mild peritumoral oedema (9). There is usually minimal spinal cord enlargement (which may be explained by the rapid and infiltrative nature of growth) which may lead to misdiagnosis as a non-neoplastic lesion.
Given that IMSCT are difficult to distinguish both clinically and radiographically, the diagnostic procedure of choice is tumour biopsy (3,11). However, surgery may be delayed given its invasive nature and inherent risks. As in this case, presumption of an autoimmune pathology given the patient’s comorbidities prompted trial of empiric treatment which led to delays in diagnosis. There is no consensus on appropriate presurgical diagnostic evaluation (3). Nevertheless, suspicion of lymphoma should prompt imaging of abdomen and chest to exclude secondary lymphoma (5).
One intervention that can assist diagnosis of lymphoma is tumour response to steroids. A marked clinical improvement or reduction in lesion contrast enhancement on neuroimaging after steroids is highly suspicious for lymphoma (3,4). The dramatic response to steroid therapy has prompted some to advise avoiding steroids until biopsy is performed to prevent obscuring definitive diagnosis (12). Surprisingly, this patient did not respond to steroids. This may have been due to the fact that he was immunosuppressed and had been on steroids before for his comorbidities.
From histopathologic analysis, the majority of cases are found to be diffuse large B cell subtype (36–57%) (4,13,14). There are atypical lymphocytes with scant cytoplasm and high mitotic activity with diffuse angiocentric infiltration and reactive changes including necrosis, oedema and gliosis (6-8,15,16). Further immunohistochemical analysis often reveals strong immunoreactivity to CD20 and CD79a (4,6,16). Other positive markers may be BCL-6, BCL-2, MUM1 (3,15). EBER-ISH positivity is associated with immunocompromised patients such as HIV and post-transplant (3,16).
Liquid biopsy is a developing field that offers a minimally invasive approach to lymphoma diagnosis. Blood samples are analysed for components of tumours including circulating tumour cells and circulating cell free nucleic acid (17). In the context of isolated PCNSL, rates of detection in peripheral blood samples are likely lower than for systemic lymphoma. A promising alternative would be analysis of cerebrospinal fluid (CSF) samples directly (18). With increasingly sensitive techniques, there is potential for invasive open biopsy to be avoided. More prospective data on the predictive value of cell free nucleic acid and standardisation of test samples is needed before this technique is employed in clinical practice (17,18).
In terms of treatment, the role of surgery is often restricted to biopsy due to the diffuse and infiltrative nature of the tumour, limiting total resection. Biopsy risks include haemorrhage and neurologic deficit (8%) (3). Attempts at resection further increases the risks of permanent neurologic dysfunction without a clear survival benefit and thus is not indicated (15). Cases where the lesion is causing compressive symptoms may benefit from decompressive surgery. This may also serve to limit progression until definitive treatment is offered (4).
In contrast to other CNS tumours, PCNSL is highly sensitive to chemotherapy and radiation therapy. There is a preference for CNS penetrating agents such as methotrexate and cytarabine. Hence, the mainstay of therapy consists of high-dose methotrexate polychemotherapy followed by adjuvant whole brain radiotherapy (15,19). Ferreri et al. [2016] demonstrated superiority of MATRix as standard chemoimmunotherapy for patients with PCNSL up to the age of 70 with response rates of 87% (19). Risks of treatment include neutropenia, thrombocytopenia, anaemia, toxicity to liver/kidney/brain, and sepsis.
Despite aggressive therapy, overall prognosis for PCNSL is still low with median survival reported to be ranging from 3 months to 17 months (4,14). Mortality at one year is as high as 26% (13). This emphasises the importance of early diagnosis and treatment, such that patients can maintain a satisfactory level of quality of life.
Conclusions
The clinical and radiological features of PCNSL are non-specific. This can make accurate diagnosis a challenge. Nevertheless, progression of disease and poor response to empiric therapy should prompt re-assessment and biopsy should be sought to prevent further delay in treatment. This patient had underlying immunosuppression and autoimmune disease. There was minimal response to steroids which is uncommon. These confounding features contributed to misdiagnosis and delay in proper treatment. Despite radiological response to chemotherapy, recurrent infections and systemic toxicity did not enable the patient to have any meaningful clinical recovery. Clinicians must keep an open mind to this rare entity, especially given its rapid progression and tendency to affect a vulnerable patient cohort.
Acknowledgments
The authors would like to thanks Dr. Fouzia Ziad, Waikato Hospital Pathologist, for contributing histology slides and pathology expertise.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-41/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-22-41/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-41/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chamberlain MC, Tredway TL. Adult primary intradural spinal cord tumors: a review. Curr Neurol Neurosci Rep 2011;11:320-8. [Crossref] [PubMed]
- Hachicha A, Belhaj A, Karmeni N, et al. Intramedullary spinal cord tumors: A retrospective multicentric study. J Craniovertebr Junction Spine 2021;12:269-78. [Crossref] [PubMed]
- Scott BJ, Douglas VC, Tihan T, et al. A systematic approach to the diagnosis of suspected central nervous system lymphoma. JAMA Neurol 2013;70:311-9. [Crossref] [PubMed]
- Guzey F, Hitay Y, Isler C, et al. Primary Spinal Intramedullary Lymphoma: A Case Report and Review of the Literature. JSM Neurosurg Spine 2015;3:1049-57.
- Sivri M, Erdoğan H, Allahverdiyev I, et al. A rare cause of spinal mass: primary intramedullary spinal cord lymphoma. Spine J 2015;15:e43-4. [Crossref] [PubMed]
- Kuhlman JJ, Alhaj Moustafa M, Gupta V, et al. Primary Cauda Equina Lymphoma Treated with CNS-Centric Approach: A Case Report and Literature Review. J Blood Med 2021;12:645-52. [Crossref] [PubMed]
- Kleinschmidt-DeMasters BK, Damek DM, Lillehei KO, et al. Epstein Barr virus-associated primary CNS lymphomas in elderly patients on immunosuppressive medications. J Neuropathol Exp Neurol 2008;67:1103-11. [Crossref] [PubMed]
- Nakamizo T, Inoue H, Udaka F, et al. Magnetic resonance imaging of primary spinal intramedullary lymphoma. J Neuroimaging 2002;12:183-6. [Crossref] [PubMed]
- Aghayev K, Vrionis F, Chamberlain MC. Adult intradural primary spinal cord tumors. J Natl Compr Canc Netw 2011;9:434-47. [Crossref] [PubMed]
- Lai R, Abrey LE, Rosenblum MK, et al. Treatment-induced leukoencephalopathy in primary CNS lymphoma: a clinical and autopsy study. Neurology 2004;62:451-6. [Crossref] [PubMed]
- Samartzis D, Gillis CC, Shih P, et al. Intramedullary Spinal Cord Tumors: Part I-Epidemiology, Pathophysiology, and Diagnosis. Global Spine J 2015;5:425-35. [Crossref] [PubMed]
- Scheichel F, Marhold F, Pinggera D, et al. Influence of preoperative corticosteroid treatment on rate of diagnostic surgeries in primary central nervous system lymphoma: a multicenter retrospective study. BMC Cancer 2021;21:754. [Crossref] [PubMed]
- Yang W, Garzon-Muvdi T, Braileanu M, et al. Primary intramedullary spinal cord lymphoma: a population-based study. Neuro Oncol 2017;19:414-21. [Crossref] [PubMed]
- Flanagan EP, O'Neill BP, Porter AB, et al. Primary intramedullary spinal cord lymphoma. Neurology 2011;77:784-91. [Crossref] [PubMed]
- Grommes C, DeAngelis LM. Primary CNS Lymphoma. J Clin Oncol 2017;35:2410-8. [Crossref] [PubMed]
- Chatterjee S, Angelov L, Ahluwalia MS, et al. Epstein-Barr virus-associated primary central nervous system lymphoma in a patient with diffuse cutaneous systemic sclerosis on long-term mycophenolate mofetil. Joint Bone Spine 2020;87:163-6. [Crossref] [PubMed]
- Cirillo M, Craig AFM, Borchmann S, et al. Liquid biopsy in lymphoma: Molecular methods and clinical applications. Cancer Treat Rev 2020;91:102106. [Crossref] [PubMed]
- Bobillo S, Crespo M, Escudero L, et al. Cell free circulating tumor DNA in cerebrospinal fluid detects and monitors central nervous system involvement of B-cell lymphomas. Haematologica 2021;106:513-21. [Crossref] [PubMed]
- Ferreri AJ, Cwynarski K, Pulczynski E, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol 2016;3:e217-27. [Crossref] [PubMed]
Cite this article as: Kong L, Raunio S. Primary central nervous system lymphoma presenting as an isolated intramedullary spinal cord lesion: a case report. AME Case Rep 2023;7:10.



