C1/C2 osteomyelitis secondary to malignant otitis externa complicated by atlantoaxial subluxation—a case report and review of the literature
Introduction
Vertebral osteomyelitis is a relatively rare condition, representing just 2.6% of all bone and joint infections. Within this group, only 10% of infections are found localised to the cervical spine (1). To the authors’ knowledge, epidemiological data on upper cervical osteomyelitis (C1-C2) currently are just limited to isolated case studies (2). Early diagnosis is challenging because these patients typically present initially with innocuous symptoms. By the time they present with neurological symptoms, the infection may have progressed to damage the odontoid ligaments, resulting in atlantoaxial subluxation, and a potential surgical emergency. In one systematic review, it was shown that neck pain featured most prominently (71–100%) in cervical osteomyelitis, followed by fever (66%), then neurologic deficits (44–59%) (3). A high index of suspicion, therefore, remains important for early diagnosis to minimise chances for a poor outcome. We present a case of C1/C2 osteomyelitis secondary to malignant otitis externa complicated by atlantoaxial instability. This case is unique because surgical management of this patient’s spinal instability was delayed, resulting in his eventual progression to cervical myelopathy. The patient’s progress, together with the challenges encountered throughout his management, are presented here. To the authors’ knowledge, this is the first reported case of a patient with septic arthritis of the atlanto-axial joint in which the infection was successfully treated with a prolonged course of antibiotics, yet continued to progress to cervical myelopathy, eventually requiring instrumented fusion of his spine.
Case presentation
Patient information
This patient was a 67-year-old male who presented to our emergency department with 1 month of progressively worsening neck stiffness and headache with fever. There was no trauma, nausea, vomiting or photophobia. His past medical history was significant for Type 2 Diabetes Mellitus which was well- controlled, and intravenous drug abuse in his youth. Prior to this, he was seen 6 months ago in an ENT clinic for severe right otalgia with purulent discharge. A diagnosis of possible right malignant otitis externa was made. He was given Polydexa ear drops (Dexamethasone + Neomycin + Polymyxin B) 2 drops twice-daily and scheduled for a computerized tomography (CT) scan, but defaulted.
Clinical findings & diagnostic assessment
On examination, his neck range-of-motion (ROM) was noted to be limited due to stiffness, Kernig’s was negative, and there was no cervical, thoracic or lumbosacral tenderness. His cranial nerves were intact, and a neurological examination was unremarkable, with 5/5 power in all myotomes and normal sensation in all dermatomes. He was noted on admission to be febrile (T 38.0 °C), initial white blood cell (WBC) of 14.52×109/L (normal 3.37×109–11.03×109/L) with 75.7% neutrophils, C-reactive protein (CRP) of 49.1 mg/L (normal 0.0–5.0 mg/L) and Erythrocyte Sedimentation Rate (ESR) of >80 mm/hr.
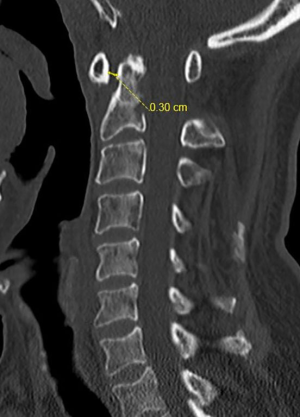
In view of his previous history, a CT scan of his temporal bones was ordered, which showed extensive bony erosions affecting the temporo-mandibular joint (TMJ) anteriorly, skull base and C1/C2. Magnetic resonance imaging (MRI) of his brain and spine was ordered as well, which showed septic arthritis of the atlantoaxial joint with atlantoaxial subluxation and an atlanto-dens interval (ADI) of 8.3 mm, resulting in compression of the high cervical cord, possible early ventriculitis and an associated pre-vertebral abscess. He was reviewed by both the ENT and spine surgeons and a diagnosis of right malignant otitis externa complicated by skull base and C1/C2 osteomyelitis, resulting in atlantoaxial instability was made and he was placed on cervical collar immobilisation (Figure 1).
Therapeutic intervention
A decision was made for a right ear tympanocentesis and myringotomy, with cervico-occipital instrumentation and decompression. Intraoperatively, frank pus was noted discharging from the eustachian tube on palpation of the post-nasal space and a decision was made to defer the spinal intervention temporarily. Cultures of the pus grew Methicillin-Sensitive Staphylococcus Aureus (MSSA), Pseudomonas Aeruginosa and Staphylococcus Lugdunensis. Post-operatively, after consultation with the Infectious Disease physicians, he was started on culture directed IV Cefepime 2 g 8-hourly and IV Metronidazole 500 mg 8-hourly, with a plan to complete 6 weeks total. However, 2 weeks into his course, he developed worsening transaminitis and generalised rashes, and the decision was made to switch to IV Piperacillin-Tazobactam 4.5 g 6-hourly. Unfortunately, within 2 weeks, he developed rashes to this too, and antibiotics was switched to IV Meropenem 1 g 8-hourly, which he was able to tolerate and complete for an additional 2 weeks. His inflammatory markers were normalising at this point: WBC 8.28×109/L (normal 3.37×109–11.03×109/L) with 67.6% neutrophils, CRP 18.8 mg/L (normal 0.0–5.0 mg/L) although ESR was still raised at >80 mm/hr. All blood cultures taken were negative as well. Therefore, we decided to switch to PO Clindamycin 500 mg 12-hourly and PO Ciprofloxacin 450 mg 6-hourly for an additional 2 more weeks. As he was improving clinically throughout, remaining afebrile, with no deterioration in neurology, a decision was made by the ENT surgeons not for operative management of his skull base osteomyelitis, and to continue with conservative management.
With regards to the atlantoaxial instability, it was decided after review by the spine surgeons that instrumentation was ill-advised in view of his cultures growing S. Aureus and P. Aeruginosa. The patient was offered a Halo-vest as an alternative, and after a discussion of the risks and benefits, was agreeable for its insertion. The Halo-vest was applied 1 week post-operatively. Unfortunately, after 11 weeks on the Halo-vest, he was no longer able to tolerate it and requested for its removal. A repeat CT cervical spine showed resolving OM changes and a reduction in his ADI back to normal (3 mm), and the Halo-vest was hence removed. He was again offered occipital-cervical fusion surgery but declined and was given an Aspen collar and discharged to outpatient follow-up (Figure 2).
Follow-up and outcomes
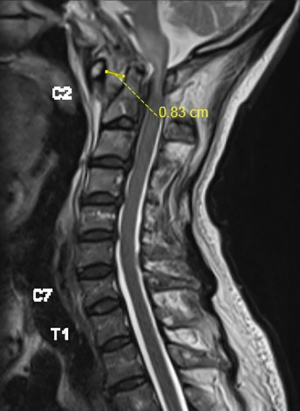
Two months later, during a follow-up clinic appointment, he complained of a left facial droop that started 1 week ago. Examination revealed an isolated lower motor neurone cranial nerve VII palsy, but an otherwise unremarkable neurological examination. He was readmitted and repeat MRI scan of the brain and neck showed new abnormal soft tissue thickening along the left Eustachian canal, extending into the left middle ear and left mastoid, with worsening skull base osteomyelitis. A CT scan of his cervical spine showed an interval worsening of the atlantoaxial subluxation with ADI now measuring 10.8 mm and soft tissue thickening in the pre-clivus and C1/C2 pre-vertebral space. His inflammatory markers, taken at the point of admission, showed WBC 7.12 (normal 3.37×109–11.03×109/L), CRP 16.4 mg/L (normal 0.0–5.0 mg/L) and ESR >80 mm/hr (Figure 3).
He then underwent a left tympanocentesis with myringotomy. Intraoperative cultures this time grew Methicillin-Resistant Staphylococcus Aureus (MRSA). He was started on PO Ciprofloxacin 750 mg 12-hourly and IV Vancomycin (titrated to keep the trough between 15-20 mg/mL) after review by the Infectious Disease physicians. He continued to decline surgical intervention throughout his hospital stay and was managed conservatively with an Aspen collar and antibiotics. However, 4 weeks into his antibiotic therapy, he again developed generalised rashes over his entire body, concerning for Red Man Syndrome. Vancomycin was therefore stopped, and he was switched to IV Daptomycin 500 mg q24h, which he tolerated without any adverse reaction. In total, he completed 11 weeks of antibiotics (4 weeks of IV Vancomycin + 7 weeks of IV Daptomycin and 11 weeks of PO Ciprofloxacin). A repeat check of his inflammatory markers at the end of antibiotic therapy showed normalisation of his WBC and CRP–WBC 5.58×109/L (normal 3.37×109–11.03×109/L), CRP 1.9 mg/L (normal 0.0–5.0 mg/L), ESR 71 mm/hr and he was subsequently discharged. Blood cultures drawn were also negative for any bacterial growth.
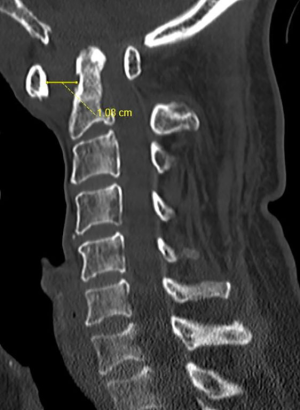
He presented again 1 month later to our Emergency Department, this time with a sub-acute, worsening weakness over both right upper and lower limbs over the past 3 weeks, and a decline in functional mobility from walking stick to wheelchair. A repeat MRI showed: worsening atlantodental subluxation (ADI 13 mm), resulting in severe spinal canal stenosis at the cervicomedullary junction and cord oedema. He was finally agreeable for surgery this time round and underwent occipital to C4 instrumentation with C1 posterior decompression. His systemic inflammatory markers at that time were: WBC 6.54×109/L (normal 3.37×109–11.03×109/L), CRP 1.4 mg/L (normal 0.0–5.0 mg/L), ESR >80 mm/hr (Figure 4).
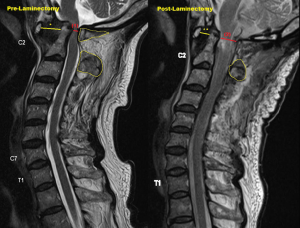
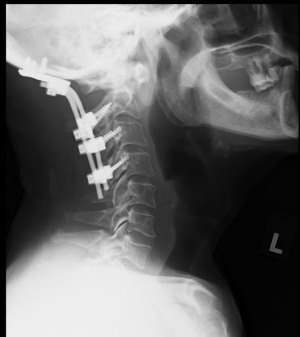
Post-operatively, he remained stable, and his power recovered to the point where he was able to ambulate with a walking frame. Objectively, power over his right C5-8 myotome recovered from 4 to 5, and his right L2-4 myotome recovered from 2 to 4. Sensation on the right C5-T1 and L2-S1 dermatome, which was previously diminished, had returned to normal as well. A repeat X-ray 2 weeks post-op showed that the implants were stable with no loosening. His intraoperative cultures did not grow any bacteria despite an extended incubation of 1 week, and he was subsequently discharged to outpatient follow-up (Figure 5).
Discussion
Challenges in diagnosis
The diagnosis of osteomyelitis at the atlantoaxial junction is difficult to make clinically, because symptoms are often non-specific. Most patients do not present initially with any neurological symptoms (4), as the development of such symptoms are oftentimes a late sequelae and a potential surgical emergency. The challenge, therefore, is in recognising a need to maintain a high index of suspicion, taking a thorough history and physical examination, with a view to early imaging should any concerns arise. Unfortunately, even when an abnormality is noted on imaging, the osteomyelitis can sometimes be misdiagnosed as a tumour, spondyloarthropathy secondary to inflammatory disease or degenerative disease (5), possibly contributing to a further delay in diagnosis. The clinician, therefore, will be required to employ all investigations available—including inflammatory markers (ESR, CRP etc.) and microbiological cultures—in addition to imaging, to finally reach the diagnosis.
With regards to the source of infection, besides direct cervical extension (i.e. from previous spinal instrumentation), the majority of cervical osteomyelitis reported seem to develop from haematogenous spread, with seeding of the capillary beds in the subchondral areas (5). In a retrospective chart review of 50 cases of vertebral osteomyelitis managed between January 2007 to December 2012 at an interregional referral centre for bone and joint infections in France (6), the source of infection was identified in 33 cases (66%), including 15 cases (30%) of urinary tract infection, 9 cases (18%) of vertebral implant infection, 5 cases (10%) of vascular catheter-related infection and 4 cases (8%) of respiratory tract infection. Contiguous spread from adjacent structures seem to be the exception, with occurrences noted only in a few case reports (7,8).
Risk factors for cervical osteomyelitis include: diabetes mellitus, solid cancer/haematologic malignancy, intravenous drug use (IVU), alcoholism, previous surgery/discography (1,3). Gram-positive organisms form the bulk of microbes isolated, with S. Aureus being the most common, followed by Streptococcus spp. Gram-negative organisms are less commonly isolated, with E. coli and Pseudomonas being the most commonly seen (9).
Taken together, the key to early diagnosis of cervical osteomyelitis is timely and adequate imaging of the cervical spine—the challenge is in deciding when imaging should be made. This can only come about through adequate history taking and physical examination, with attention to the patient’s comorbidities and risk factors, with the assistance of biochemical markers of inflammation and microbiological cultures. In the case of our patient, early imaging was prompted in view of his chronic, progressive symptoms (neck pain and fever), in addition to several risk factors (DM, previous IVU, recent inadequately treated malignant otitis externa prior to the onset of his presentation of fever and neck pain).
Challenges in management
To date, management of vertebral osteomyelitis remains controversial, partly because of limited evidence for the many treatment options available. The recently published Infectious Diseases Society of America (IDSA) guidelines are based largely on expert opinion and observational studies (10). Indications for non-surgical management of cervical osteomyelitis include absence of neurological deficit, good pain control with analgesic medications, no associated epidural abscess or treatment deformity (3). Yet, the optimal duration of antimicrobial therapy remains controversial. Some authors propose 6-8 weeks of parental antibiotic therapy alone, while others advocate following this with 2 or more months of oral therapy (11). The IDSA guidelines published in 2015 recommended a duration of 6 weeks of parenteral administration for bacterial spinal infection. This was based on a single randomised control trial (n=359), which showed non-inferiority in patients receiving either 6 or 12 weeks of parenteral antibiotics (12). Cure rates were reported as 90.9% in both the 6- and 12-week groups at the end of 1 year and the failure rate cited for most studies have varied between 10–30% (10).
Taken together, these suggest that conservative management of vertebral osteomyelitis is not always successful. Factors associated with worse outcome are not yet well defined, but may include multidisc disease, the presence of concomitant epidural abscess, old age, significant comorbidities or immunosuppression (10).
One of the challenges faced in the management of this patient was in determining the optimal duration of antibiotic therapy. In his first presentation with fever, headache and neck stiffness, he was given a total of 6 weeks of IV antibiotics (2 weeks of Cefepime and Metronidazole, 2 weeks of Piperacillin-Tazobactam and 2 weeks of Meropenem), followed by 2 weeks of oral antibiotics (Clindamycin and Ciprofloxacin). His inflammatory markers before and towards the end of antibiotic therapy were trending downwards, although they had not entirely normalised (WBC 14.52 to 8.28×109/L; CRP 49.1 to 18.8 mg/L; ESR persistently >80 mm/hr). Nevertheless, the decision was made to stop antibiotics as he remained afebrile and neurologically stable throughout the duration of therapy. Furthermore, a repeat CT scan done 3 weeks after antibiotics showed radiological evidence of improvement in his OM.
Unfortunately, it became evident that this duration of antibiotic therapy was inadequate to treat his infection when he presented 2 months later with a left lower motor facial nerve palsy due to a progression of his infection into the contralateral ear. This was confirmed with bacterial cultures taken from samples of his left middle ear effusion, which grew MRSA (likely from previous prolonged use of antibiotics). Inflammatory markers repeated at the time of readmission showed that his CRP and ESR had not entirely normalised (WBC 7.12×109/L, CRP 16.4 mg/L and ESR >80 mm/hr). He was therefore given another 11 weeks of antibiotics (4 weeks of IV Vancomycin + 7 weeks of IV Daptomycin and 11 weeks of PO Ciprofloxacin), and it was only then that his CRP normalised, although his ESR continued to remain high (CRP 1.9 mg/L, ESR 71 mm/hr).
The IDSA has made a weak recommendation based on low quality evidence to monitor ESR and CRP on patients on antibiotic therapy and suggested that patients with at least a 25—33% in systemic inflammatory markers after 4 weeks of antibiotics may be at reduced risk of treatment failure (10,13). In one study, it was found that after 4 weeks of treatment, ESR values >50 mm/hr and CRP values >27.5 mg/L may confer a significantly higher risk of treatment failure (14). However, it should also be noted that patients in whom systemic inflammatory markers continue to be high can still have successful outcomes, highlighting the poor specificity of these markers (10,13). There is still a need therefore, for these values to be interpreted in the clinical context of the patient.
The second challenge faced in the management of this patient was in determining the optimal timing of surgery. Indications for surgery include progressive neurologic deficits, progressive deformity and spinal instability with or without pain despite adequate antimicrobial therapy (10). However, even though our patient remained neurologically stable throughout the time he was on antibiotics, in the setting of C1/2 osteomyelitis with a widened ADI (typically taken to be >3.5 mm), the spine will still be unstable as the odontoid ligaments have likely been damaged. Definitive fixation of his spine was initially delayed due to concerns of instrumenting infected bone and causing a layer of biofilm to form on the implants, which would have been difficult to eradicate. However, some studies have shown that spinal implants can be inserted safely even in patients with deep spine infection and may not be as dangerous as previously thought (15,16). One study even reported successful outcomes in instrumenting immunocompromised patients with vertebral osteomyelitis in the setting of active infection (17). Surgery would also have allowed achievement of better source control of the infection by means of debriding infected tissue. Thus, had surgery been performed early on in his first admission, it may have been possible to prevent his subsequent two readmissions, a prolonged course of antibiotics, and his eventual development of cervical myelopathy from worsening instability.
Finally, in the setting of spinal instability, definitive surgical fixation may still be warranted despite adequate treatment of the infection with antibiotics. This patient was initially managed with a Halo vest, which he was compliant to for 11 weeks, and subsequently an Aspen collar, but this still resulted in a poor outcome. Even though his infection was successfully treated with antibiotics, as evidenced by his normalised inflammatory markers (WBC and CRP) at the end of his 2nd admission, he presented again to us a 3rd time with cervical myelopathy (subacute, worsening right-sided weakness over 3 weeks) and severe spinal canal stenosis secondary to atlantoaxial subluxation. During this admission, there was no evidence of a recurrence of the infection on imaging, systemic inflammatory markers and bacterial cultures.
With regards to surgical treatment, several treatment strategies have been described, including an anterior or posterior approach, single vs staged surgery, with or without instrumentation (18). None of these treatment strategies have been subjected to randomised controlled trials yet. Nevertheless, most authors continue to favour surgical treatment, especially in cases of doubt or progression. This is especially so as overall mortality is lower in operated patients, and delayed surgical treatment often entails significantly poorer surgical outcome (19). In the case of our patient, early surgical treatment was delayed in part because of patient factors, although fortunately for him, we were able to reverse his neurological deficit before it became permanent.
Conclusions
This case serves to illustrate the potential challenges in the diagnosis and management of cervical osteomyelitis. Firstly, the initial presenting symptoms are often non-specific. The clinician therefore needs to maintain a high index of suspicion and use all available means of investigations, including serum inflammatory markers, bacterial cultures and spinal imaging in order to arrive at the diagnosis. Secondly, there is no good evidence base for optimal duration of antibiotics for patients with cervical osteomyelitis. While there is some evidence for the use of monitoring systemic inflammatory markers, they are not highly specific, and the clinician would still be required to interpret this in the context of the patient. Finally, if non-operative management is attempted, the separate issue of spinal instability must be kept in mind, and the patient may still be a candidate for surgical fixation in the future.
Patient perspective
We had great difficulty in convincing the patient to undergo surgical fixation of his spine in the early course of his disease. This was because even though he had radiological evidence of spinal instability, there was no impairment in his neurology or functional mobility at that time. He therefore felt that surgical fixation then would offer him no tangible benefits and expose him to risks that he was not prepared to take. He understood that there was a risk he could eventually progress to paralysis and said that he would ‘leave it to God’ and deal with it ‘when the time comes’. Furthermore, he felt that losing the ability to flex his neck after surgical fixation was unacceptable as he was a religious person and valued the ability to bow his head during prayers. It was only after developing cervical myelopathy, when he understood what it meant to be wheelchair bound and saw how much difficulty his wife had in assisting him with transfers, was he finally agreeable for surgery. He has remained happy and satisfied with the outcome of the surgery since then.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/acr.2020.03.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Doutchi M, Seng P, Menard A, et al. Changing trends in the epidemiology of vertebral osteomyelitis in Marseille, France. New Microbes New Infect 2015;7:1-7. [Crossref] [PubMed]
- Al-Hourani K, Al-Aref R, Mesfin A. Upper Cervical Epidural Abscess in Clinical Practice: Diagnosis and Management. Global Spine J 2016;6:383-93. [Crossref] [PubMed]
- Barnes B, Alexander JT, Branch CL. Cervical osteomyelitis: a brief review. Neurosurg Focus 2004;17:E11. [Crossref] [PubMed]
- Vakili M, Crum-Cianflone NF. Spinal Epidural Abscess: A Series of 101 Cases. Am J Med 2017;130:1458-63. [Crossref] [PubMed]
- Lam CH, Ethier R, Pokrupa R. Conservative therapy of atlantoaxial osteomyelitis. A case report. Spine (Phila Pa 1976) 1996;21:1820-3. [Crossref] [PubMed]
- Doutchi M, Seng P, Menard A, et al. Changing trends in the epidemiology of vertebral osteomyelitis in Marseille, France. New Microbes New Infect 2015;7:1-7. [Crossref] [PubMed]
- Radhiana H, Win Mar Salmah J. C1 and C2 vertebrae osteomyelitis: a misleading presentation leading to a fatal outcome. Int Med J Malaysia 2009;8:49-51.
- Paul CA, Kumar A, Raut VV, et al. Pseudomonas cervical osteomyelitis with retropharyngeal abscess: an unusual complication of otitis media. J Laryngol Otol 2005;119:816-8. [Crossref] [PubMed]
- Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev 2000;23:175-204. [Crossref] [PubMed]
- Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis 2015;61:e26-46. [Crossref] [PubMed]
- Nagashima H, Tanishima S, Tanida A. Diagnosis and management of spinal infections. J Orthop Sci 2018;23:8-13. [Crossref] [PubMed]
- Bernard L, Dinh A, Ghout I, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet 2015;385:875-82. [Crossref] [PubMed]
- Carragee EJ, Kim D. The clinical use of erythrocyte sedimentation rate in pyogenic vertebral osteomyelitis. Spine (Phila Pa 1976) 1997;22:2089-93. [Crossref] [PubMed]
- Yoon SH, Chung SK, Kim KJ, et al. Pyogenic vertebral osteomyelitis: identification of microorganism and laboratory markers used to predict clinical outcome. Eur Spine J 2010;19:575-82. [Crossref] [PubMed]
- Hey HWD, Ng LWN, Tan CS, et al. Spinal implants can be inserted in patients with deep spine infection: results from a large cohort study. Spine (Phila Pa 1976) 2017;42:E490-5. [Crossref] [PubMed]
- Rayes M, Colen CB, Bahgat DA, et al. Safety of instrumentation in patients with spinal infection: clinical article. J Neurosurg Spine 2010;12:647-59. [Crossref] [PubMed]
- Carragee E, Iezza A. Does acute placement of instrumentation in the treatment of vertebral osteomyelitis predispose to recurrent infection: long-term follow-up in immune compromised patients. Spine (Phila Pa 1976) 2008;33:2089-93. [Crossref] [PubMed]
- Chen WH, Jiang LS, Dai LY. Surgical treatment of pyogenic vertebral osteomyelitis with spinal instrumentation. Eur Spine J 2007;16:1307-16. [Crossref] [PubMed]
- Alton TB, Patel AR, Bransford RJ, et al. Is there a difference in neurologic outcome in medical versus early operative management of cervical epidural abscesses?. Spine J 2015;15:10-7. [Crossref] [PubMed]
Cite this article as: Low G, Leong A, George R, Tan G. C1/C2 osteomyelitis secondary to malignant otitis externa complicated by atlantoaxial subluxation—a case report and review of the literature. AME Case Rep 2020;4:19.