
Anal squamous cell carcinoma with metastasis to duodenum causing duodenal stricture and gastric outlet obstruction
Introduction
Anal cancer accounts for 2.5% of all digestive system malignancies with an incidence of around 8,600 annually (1). A higher incidence has been associated with females, human papillomavirus infection (HPV), multiple sexual partners, genital warts, cigarette smoking, anal intercourse, and HIV infection (2). Primary rectal squamous cell carcinomas (SCC) are very rare tumors accounting for only 0.25% of all rectal carcinomas with less than 80 reported cases in English literature (3-6).
Involvement of the small bowel is not uncommon in the context of widespread peritoneal carcinomatosis (7,8). Anal cancer is primarily a loco-regional disease, which rarely metastasizes (9). In the event of metastasis from primary SCC of the anal canal, the most common sites of extra-pelvic metastases are the liver, lungs and extra-pelvic lymph nodes, although spread to the peritoneum, bone and other sites may also occur (7-9). However, spread of the metastatic disease to the small intestine is rare (7,8). To our knowledge, metastatic spread from primary anal SCC to small bowel has been reported only once before (9). We report an unusual case of SCC of the anal canal with duodenal metastases in a 49-year-old female who had presented with symptoms of persistent nausea and vomiting eight months after the primary diagnosis.
Case presentation
A 49-years-old female with an 8-month history of anal SCC Stage IIIA, status post chemo-radiation therapy and status post loop ileostomy creation was seen for complaints of vague abdominal pain, anorexia, nausea, and vomiting. She had been experiencing diffuse abdominal pain, non-radiating, worst postprandial, with associated anorexia, nausea, and non-bloody, daily emesis of food contents for two to three weeks duration. On physical examination, she appeared comfortable and vital signs were within normal limits. Her examination was significant for mild periumbilical tenderness to deep palpation.
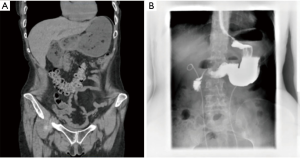
CT of the abdomen and pelvis with intravenous contrast revealed distended stomach with food contents and distended proximal duodenum (Figure 1) with normal appearing third portion of the duodenum (Figure 1A) without any enlarged lymph nodes or evidence of liver lesions. Esophagogastroduodenoscopy (EGD) revealed moderate gastritis with duodenal edema resulting in the narrowing of the second portion of duodenum indicative of duodenal stricture (Figure 2A,B). An upper gastrointestinal series revealed distended stomach with air-fluid level and marked narrowing at the junction of the distal descending and transverse duodenum with “U-shaped” appearance with normal appearing jejunum (Figure 1B). Colonoscopy was performed via the stoma showing normal colon proximal to diverting colostomy, normal terminal ileum, narrowed and scarred rectal vault to approximately 20 cm proximal to anal verge (Figure 2C,D).
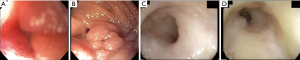
A biopsy from the duodenal stricture revealed duodenal mucosa with scattered malignant cells and malignant cell clusters within lymphatic spaces, consistent with metastatic carcinoma (Figure 3A,B). Immunohistological staining demonstrated malignant cells positive for CK7, p16, p63 and negative for CK20, CDX2, GATA-3, CA19.9, TTF-1, Napsin A and PAX-8 favoring a metastatic anal SCC given the patient's history of anal cancer (Figure 3C,D,E). A gastric biopsy was significant for chronic focally active antral gastritis with reactive changes and focal foveolar hyperplasia with immunological staining negative for Helicobacter pylori. CT of the chest was negative for any metastatic spread or any lung involvement.

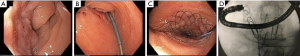
Subsequent therapeutic EGD identified a duodenal mass at the 2nd portion of the duodenum. A guidewire was successfully passed through the stenotic lumen under fluoroscopic guidance and a 22×60 wallflex duodenal stent was deployed across the area of luminal narrowing (Figure 4A,B,C). The position of the stent was confirmed under fluoroscopic guidance (Figure 4D).
Discussion
Primary rectal SCC are very rare tumors. Additionally, either primary or metastatic SCC of the gastrointestinal tract is extremely rare, with very few reported cases in the literature. Rarely, the small intestine can be a site of secondary deposits from primaries originating in the lung, cervix, kidney, and melanoma (10,11).
In our case, the patient with an established 8-month history of anal SCC stage IIIA who was status post radiation, chemotherapy, and status post loop ileostomy creation presented with a rare duodenal metastasis, which has only been reported once before to our knowledge (9). The possibility of primary SCC of the small bowel was excluded given the duodenal stricture biopsy showing duodenal mucosa with scattered malignant cells within lymphatic spaces, consistent with metastatic carcinoma with immunohistological staining demonstrating malignant cells positive for CK7, p16, p63 and negative for CK20, CDX2, GATA-3, CA19.9, TTF-1, Napsin A and PAX-8, favoring a metastatic lesion.
The route of metastasis to the duodenum depends upon the site of the primary lesion. The different mechanisms postulated for small bowel metastasis from abdomen or pelvis include; retrograde lymphatic spread following initial blockade of para-aortic or mediastinal lymph nodes, peritoneal seedlings, direct extension by continuity or by permeation of the lymphatic spaces in connective tissues and hematogenous route (12). In our case, lymphatic spread appeared to be the most likely mode of spread due to the widespread malignant cell clusters within lymphatic spaces on histopathology.
Radiotherapy with concurrent chemotherapy is the standard of care for patients with nonmetastatic squamous cell anal cancer (13). Historically, these patients were treated mainly by surgical resection, similar to rectal adenocarcinoma. However, recently, several retrospective case series demonstrated good results with definitive chemoradiotherapy (4,13-16). Patients with metastatic disease have a poor prognosis, with 5-year median overall survival rates of 10% in men and 20% in women and the standard systemic treatment of metastatic lesions is cisplatin and 5-fluorouracil (17). In general, metastatic lesions to the small bowel from primaries originating in the lung, breast, hepatocellular carcinoma (HCC), and melanoma are often palliative (4,14-16).
Our patient's unique presentation makes this case noteworthy. As a result of this case, for the first time, we are establishing a unique site of metastasis for anal SCC. Our patient presented with symptoms of post-prandial abdominal pain, nausea, vomiting, and anorexia, consistent with gastric outlet obstruction. Therefore, it is important for clinicians to pay attention to such symptoms in consideration for a possible metastatic lesion to the small bowel from primaries such as the lung, breast, HCC, melanoma, and now anal SCC.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Palefsky JM. Anal human papillomavirus infection and anal cancer in HIV-positive individuals: an emerging problem. AIDS 1994;8:283-95. [Crossref] [PubMed]
- Williams GT, Blackshaw AJ, Morson BC. Squamous carcinoma of the colorectum and its genesis. J Pathol 1979;129:139-47. [Crossref] [PubMed]
- Clark J, Cleator S, Goldin R, et al. Treatment of primary rectal squamous cell carcinoma by primary chemoradiotherapy: should surgery still be considered a standard of care? Eur J Cancer 2008;44:2340-3. [Crossref] [PubMed]
- Rasheed S, Yap T, Zia A, et al. Chemo-radiotherapy: an alternative to surgery for squamous cell carcinoma of the rectum--report of six patients and literature review. Colorectal Dis 2009;11:191-7. [Crossref] [PubMed]
- Wang ML, Heriot A, Leong T, Ngan SY. Chemoradiotherapy in the management of primary squamous-cell carcinoma of the rectum. Colorectal Dis 2011;13:296-301. [Crossref] [PubMed]
- Kadakia SC, Parker A, Canales L. Metastatic tumors to the upper gastrointestinal tract: endoscopic experience. Am J Gastroenterol 1992;87:1418-23. [PubMed]
- Richie RE, Reynolds VH, Sawyers JL. Tumor metastases to the small bowel from extra-abdominal sites. South Med J 1973;66:1383-7. [Crossref] [PubMed]
- Farmer RG, Hawk WA. Metastatic tumors of the small bowel. Gastroenterology 1964;47:496-504. [Crossref] [PubMed]
- Abdulmajed M, Ghalib A, Mohamed M, et al. Intestinal metastasis from primary epidermoid anal carcinoma in a 34 year old male presented with acute bowel obstruction. J Surg Case Rep 2012;2012:1. [Crossref] [PubMed]
- John AK, Kotru A, Pearson HJ. Colonic metastasis from bronchogenic carcinoma presenting as pancolitis. J Postgrad Med. 2002;48:199-200. [PubMed]
- Arulraj P, Damodaran V, Raman ML, et al. Small bowel metastases from esophageal and oropharyngeal cancers. Indian J Gastroenterol 2005;24:116-8. [PubMed]
- Das P, Crane CH, Eng C, et al. Prognostic factors for squamous cell cancer of the anal canal. Gastrointest Cancer Res 2008;2:10-4. [PubMed]
- Li Y, Wang J, Ma X, et al. A review of Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Int J Biol Sci 2016;12:1022-31. [Crossref] [PubMed]
- Takeda M, Kawahara H, Ogawa M, et al. Reevaluation of Preoperative Chemoradiotherapy for Clinical T3 Lower Rectal Cancer: A Multicenter Collaborative Retrospective Clinical Study. Anticancer Res 2019;39:3047-52. [Crossref] [PubMed]
- Park JS, Park SY, Kim HJ, et al. Long-term Oncologic Outcomes After Neoadjuvant Chemoradiation Followed by Intersphincteric Resection With Coloanal Anastomosis for Locally Advanced Low Rectal Cancer. Dis Colon Rectum 2019;62:408-16. [Crossref] [PubMed]
- Dewdney A, Rao S. Metastatic Squamous Cell Carcinoma of the Anus: Time for a Shift in the Treatment Paradigm? ISRN Oncol 2012;2012:756591. [Crossref] [PubMed]
Cite this article as: Yuridullah R, Kaur P, Estifan E, Sanchez J, Nanavati S, Singhal M. Anal squamous cell carcinoma with metastasis to duodenum causing duodenal stricture and gastric outlet obstruction. AME Case Rep 2019;3:33.


