A case report and literature review: incidental jejunal ectopic pancreatic tissue in an emergency bowel exploration for suspected intussusception
Introduction
Ectopic pancreatic (EP) tissue is a congenital abnormality whereby pancreatic tissue is found outside of the anatomical confines of the pancreas (1,2). Although heterotopic pancreatic tissue can be present anywhere in the abdominal cavity, it is usually found in the upper gastrointestinal tract, most commonly the stomach (1,3). The true incidence and prevalence are not known as most individuals are asymptomatic, and hence most cases of EP are found incidentally upon performing a procedure for another purpose (1-3). Patients that are symptomatic usually experience non-specific symptoms that are related to the anatomical location of the ectopic pancreas (4). The preoperative diagnosis of EP remains challenging, with limited imaging options to point to the specific diagnosis. Surgical excision is potentially curative for EP (5). Surgical excision can provide tissue for histological diagnosis and can serve to rule out malignancy (2). Additionally, surgical excision may prevent future complications EP pancreatitis, pseudocyst formation, and malignant transformation (1,2,6).
Case presentation
A 62-year-old female, known case of hypertension, dyslipidemia, and glucose-6-phosphate dehydrogenase (G6PD) deficiency was admitted to our institution as a newly diagnosed case of acute myelocytic leukemia. During her admission she started to experience abdominal pain on day 9 of induction chemotherapy with FLAG protocol (Flutarubicin/Cytarabine). The patient was also kept on broad antibiotic coverage prophylactically.
The general surgery team were consulted on day 11 after the pain progressively increased over the previous 3 days despite conservative management. It was progressive, generalized, and colicky in nature. It was associated with 3–4 episodes of watery non-bloody loose stools daily over 4 days and 3 episodes of vomiting in the last 2 days, green in colour. The patient spiked a fever twice on day 10 of chemotherapy. No urinary symptoms were present.
On examination, there was mild generalized tenderness in the abdomen with no guarding or rigidity. Rebound tenderness was negative. On auscultation normal bowel sounds were heard. Per rectal examination revealed green stained stool and an empty rectum. The patient’s vitals were as follows: heart rate 142 bpm, blood pressure 131/84 mmHg, respiratory rate 22, temperature 37.5 °C.
Upon investigation, complete blood count (CBC) was obtained showing a white blood cell (WBC) count of 0.13×10^9/L, haemoglobin 8.6 g/dL, and platelet count of 22×10^9/L, albumin 27.4 g/L. The patient was having hypokalemia and hypomagnesemia which were corrected with IV fluids. Remaining electrolytes, renal function tests, and liver function tests were normal. The patient was on Caspofungin 50 mg q24, Augmentin 1 g q12, Azithromycin 250 mg q24, Valcyclovir 500 mg q24.
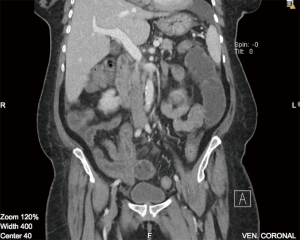
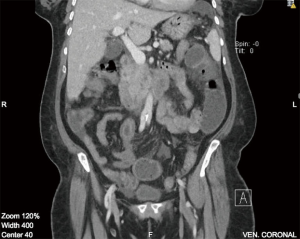
On imaging, plain film abdomen showed dilated small bowel loops and multiple air fluid levels on erect view. Chest X-ray showed no free air under the diaphragm. Computed tomography (CT) of the abdomen and pelvis with IV and oral contrast was done showing prominent dilatation of the jejunal small bowel segments, compatible with obstruction. On the jejunoileal junction and proximal ileal segments there was appearance of intussusception and thickened wall of the ileal segment (6 cm thickness) (Figure 1). Distal ileal and colonic segments were collapsed. There was perihepatic, perisplenic and intrapelvic free fluid. Furthermore, there was a small amount of free air focus in the subhepatic area (Figure 2) with bilateral small pleural effusions. No suspicious lymph nodes were detected.
Stool analysis was done which was negative for Clostridium difficile. On stool culture no bacterial growth was detected after 48 hours of aerobic and anaerobic incubation.
The patient was scheduled for an urgent diagnostic laparoscopy bowel exploration as indicated by the CT findings of free air under the diaphragm and suspicion of intussusception in an immunocompromised patient. An alternative diagnosis considered was that of neutropenic typhlitis, which has similar symptoms, CT findings and presents in severely immunocompromised and myelosuppressed patients. Preoperatively, the patient was given 2 units of irradiated platelet apheresis, 1 unit of packed red blood cells (PRBC) and received an immediate dose of Neupogen 300 mcg.
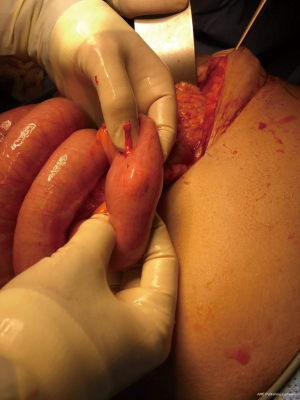
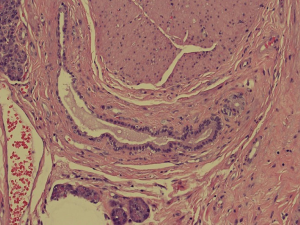
During the operation the bowel was found to be dilated and edematous. A free bile stained fluid was seen. The decision was made to convert into an open laparotomy via small upper midline incision as a result of iatrogenic bowel perforation of the edematous bowel. The fluid was aspirated. There was a perforation at 100 cm from the ileocecal junction which was sutured. No intussusception was found at the time of exploration and no transition zone could be elicited, healthy bowel was seen. A small tan coloured mass that was soft in consistency was seen in the antimesenteric area, measuring 3×3, 30 cm from the duodenojejunal junction (Figure 3). The mass was excised via wedge excision due to the small mass circumference and the high risk of intestinal leakage post excision anastomosis in this patient. It was then sent for histopathology. The patient tolerated the procedure well and left the operating room in good condition.
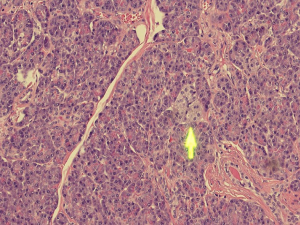
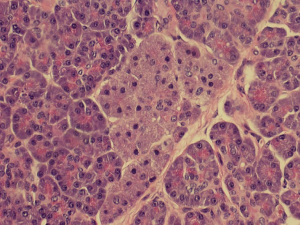
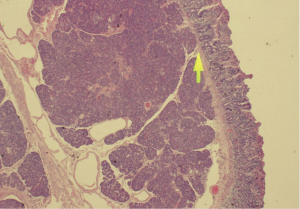
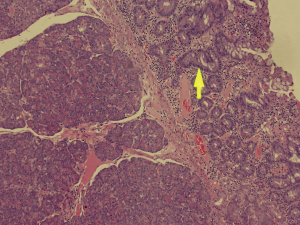
Histopathological analysis of the specimen revealed complete excision of the mass, which was reported to be benign pancreatic tissue, showing both endocrine and exocrine elements (islets of Langerhans, pancreatic acini, and pancreatic ducts) (Figures 4-9). No atypical features were seen and there was no evidence of malignancy.
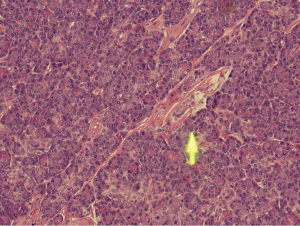
Post-operatively the patient was admitted to the intensive care unit (ICU) for observation. She made a good recovery and was later discharged with outpatient follow-up in the general surgery and haematology clinics. She was seen in the surgery clinic a week after discharge and was doing well. On follow-up a week later, the wound looked hyperaemic and infected with minimal discharge. Wound culture was taken and the patient was covered with empiric antibiotics.
Discussion
Heterotopic pancreas (HP) is defined as pancreatic tissue which is anatomically separate from the main pancreas and is devoid from its vascular or ductal continuity (1,2). It is also referred to as ectopic pancreas, accessory pancreas, aberrant pancreas or pancreatic choristoma (1,2). It was first reported in medical literature in 1729 (7).
While the aetiology of HP remains unknown, the sites in which HP can be found are partially explained by the most widely accepted embryological theory which is the “misplacement theory”. According to this theory, along the path of embryologic development of the pancreas, as the ventral and dorsal parts of pancreas approximate and fuse, small deposits of pancreatic tissue are dropped in the foregut of the fetus. These tissues later on develop into mature HP in the gastrointestinal tract (8). Other less favourable theories include the metaplasia theory and the totipotent cell theory (1-3).
The histological morphology of EP tissue can be classified according to Heinrich’s criteria, proposed in 1909 (9). Type 1 EP tissue contains cells of exocrine glands, excretory ducts and islets of Langerhans; type 2 contains only excretory glands and excretory ducts; type 3 contains only excretory ducts (8). In 1973, Gasper-Fuentes proposed a modified version of the Heinrich criteria, comprising of four types of EP tissue:
- Type I: typical pancreatic tissue composed of acini, ducts and islet cells;
- Type II: the canalicular variety composed of pancreatic ducts only;
- Type III: exocrine pancreas composed of acinar tissue only;
- Type IV: endocrine pancreas—islet cells only.
The patient reported herein is classified under type I.
The true incidence and prevalence are hard to determine and remain unknown as most affected individuals are asymptomatic. The incidence of EP in autopsies is reported to be 0.5–13.7% (1,2), with a male predilection (9). Most cases of EP are found incidentally upon performing a procedure for another purpose, most commonly laparotomy. It has been reported that EP is incidentally found in 0.2% of laparotomies (1-3), with some papers reporting that figure at 0.5% (4) in upper laparotomies. Around 31% of all ectopic pancreatic (EP) tissue reported is detected intra-operatively during procedures for other conditions (10). HP may be encountered at any age, but it most commonly presents in the 5th and 6th decades of life (3).
It is reported that 70–90% of EP occurs in the upper gastrointestinal tract (7). The common location for HP to be found is in the stomach (25–38%) followed by duodenum (17–21%) and jejunum (15–21%). Less common sites are esophagus, ileum and Meckel’s diverticulum. Rarer sites include gallbladder, omentum, periampullary region, umbilicus, spleen, bile ducts, mediastinum and fallopian tubes (1,3).
Although most cases are clinically asymptomatic, some patients may complain of non-specific symptoms which are mostly dependent on the location of the HP. A series of 34 histologically verified cases found that 38% of EP tissue is symptomatic (11). Another study reported that 33–47% of EP is symptomatic with varied clinical features depending on the location of the lesion, its size and whether it is concurrent with another condition (4).
Oesophageal EP may cause dysphagia and epigastric pain as its predominant symptoms. A lesion at the gastric pylorus may cause gastric obstruction. Jejunal HP may serve as a precipitating factor for the development of intussusception and hence obstruction (1,12). In our case we hypothesize that the EP tissue may have been the leading point of an intussusception. A mass in the gallbladder may cause obstructive jaundice (5). Other non-specific symptoms may include bleeding from the gastrointestinal tract, chronic abdominal pain and weight loss (1,3). These symptoms may be difficult to recognize as some cases may present with other concurrent comorbidities such as in our case, masking the symptoms (if any) that might be experienced by the patent.
The pre-operative diagnosis of HP remains difficult. There are generally no biochemical markers to diagnose HP (5). Few investigation methods have been proposed in the literature to aid the diagnosis of HP pre-operatively. These include endoscopy +/− ultrasound, CT, barium studies, magnetic resonance imaging (MRI) and magnetic resonance cholangiopancreatography (MRCP) (3,12). However, a definitive diagnosis can only be established upon histological examination of the suspected lesion (2,3,12).
Endoscopic ultrasound with or without biopsy has been used in few cases where HP tissue was found in the stomach. Unfortunately, in the majority of cases the biopsy obtained returned with inconclusive results (13). Visualisation of central umbilication, which is a characteristic feature of HP, may be appreciated more often with endoscopy rather than radiologic imaging (3). On endoscopic ultrasound, HP may appear as a solid submucosal mass of low echogenicity when compared to the hyperechoic submucosa, and isoechoic to the hypoechoic muscularis propria (14).
A previous study has shown that HP tissue may be visualised by CT as being isodense to the native pancreas (3). However, these results are not specific and cannot differentiate the lesion from other gastrointestinal tumours (12). Jejunal HP, such as in our case report, has a few differences from HP in other locations such as gastric or duodenal lesions. Firstly, jejunal lesions have not been reported to show features of umbilication or hyperenhancement, rendering the diagnosis of HP in the jejunum by CT alone extremely difficult (12,15). Secondly, endoluminal growth pattern are associated with (82–87%) of gastric and duodenal lesions while no dominant growth pattern has been observed in jejunal lesions, with lesions showing endoluminal, extraluminal and mixed growth patterns equally (12,15). Furthermore, it has been reported that barium studies were able to identify central indentation or umbilication in some cases of small bowel HP (15).
MRI is very useful for the diagnosis of HP. HP demonstrates a characteristic high signal intensity at T1-weighted magnetic resonance (MR), thus it is highly useful in differentiating HP from other lesions. The “ectopic duct” sign, a dilated EP duct, is a useful finding that could be appreciated on both T2-weighted MR and MRCP. The use of secretin during MRCP may further improve the visualization of this sign (14). In addition, the appearance of HP mimics that of the native pancreas, as it is isointense with the native pancreas in all MRI sequences (1,3).
Surgical excision is potentially curative for HP, with local excision being preferred over radical surgery (5). Surgical excision will also provide tissue for histological diagnosis and evidence to rule out malignancy (2). Additionally, surgical excision may prevent future complications. General complications of HP are similar to those of the orthotopic pancreas and include pancreatitis, pseudocyst formation, and malignant transformation (1,2). There have been reports of intraductal papillary mucinous neoplasms that have arisen from the malignant transformation of EP tissue (6). Malignant transformation of HP is rare (0.7–1.8% of cases) and is estimated to be similar in frequency as that of the native gland (3,16).
Conclusions
In conclusion, although pancreatic heterotopia is rare, it should be considered in the differential diagnosis of diagnostically challenging cases. Despite the development of modern diagnostic modalities, its diagnosis continues to be a challenging one. Surgical excision provides symptomatic relief and is recommended especially if diagnostic uncertainty remains or in situations where EP tissue is encountered incidentally during abdominal laparoscopies or laparotomies. The possibility of complications arising including ectopic tissue pancreatitis, pseudocyst formation, and malignant transformation of EP tissue further support the case for excision of EP tissue that is found incidentally.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent for the reporting of this case report was obtained from the patient.
References
- Rezvani M, Menias C, Sandrasegaran K, et al. Heterotopic Pancreas: Histopathologic Features, Imaging Findings, and Complications. Radiographics 2017;37:484-99. [Crossref] [PubMed]
- Sathyanarayana SA, Deutsch GB, Bajaj J, et al. Ectopic pancreas: a diagnostic dilemma. Int J Angiol 2012;21:177-80. [Crossref] [PubMed]
- Uslu N. Ectopic (heterotopic) pancreas in the mesentery of the jejunum: Imaging findings. CRCM 2013;2:277-80. [Crossref]
- So HF, Cross TJ, Zonta M. A case report of incidental ectopic pancreatic tissue during laparoscopic appendicectomy. Int J Surg Case Rep 2018;45:77-8. [Crossref] [PubMed]
- Erkan N, Vardar E, Vardar R. Heterotopic pancreas: report of two cases. JOP 2007;8:588-91. [PubMed]
- Okamoto H, Fujishima F, Ishida K, et al. Intraductal papillary mucinous neoplasm originating from a jejunal heterotopic pancreas: report of a case. Surg Today 2014;44:349-53. [Crossref] [PubMed]
- Cano DA, Hebrok M, Zenker M. Pancreatic development and disease. Gastroenterology 2007;132:745-62. [Crossref] [PubMed]
- Kilius A, Samalavicius NE, Danys D, et al. Asymptomatic heterotopic pancreas in Meckel's diverticulum: a case report and review of the literature. J Med Case Rep 2015;9:108. [Crossref] [PubMed]
- Christodoulidis G, Zacharoulis D, Barbanis S, et al. Heterotopic pancreas in the stomach: a case report and literature review. World J Gastroenterol 2007;13:6098-100. [Crossref] [PubMed]
- Zhang Y, Sun X, Gold JS, et al. Heterotopic pancreas: a clinicopathological study of 184 cases from a single high-volume medical center in China. Hum Pathol 2016;55:135-42. [Crossref] [PubMed]
- Armstrong CP, King PM, Dixon JM, et al. The clinical significance of heterotopic pancreas in the gastrointestinal tract. Br J Surg 1981;68:384-7. [Crossref] [PubMed]
- Watanabe M, Shiozawa K, Kishimoto Y, et al. Heterotopic Pancreas of the Jejunum Incidentally Detected by Preoperative Abdominal CT: Report of Two Cases and Review of the Literature. Case Rep Gastroenterol 2012;6:576-82. [Crossref] [PubMed]
- Trifan A, Târcoveanu E, Danciu M, et al. Gastric heterotopic pancreas: an unusual case and review of the literature. J Gastrointestin Liver Dis 2012;21:209-12. [PubMed]
- Kung JW, Brown A, Kruskal JB, et al. Heterotopic pancreas: typical and atypical imaging findings. Clin Radiol 2010;65:403-7. [Crossref] [PubMed]
- Kim DW, Kim JH, Park SH, et al. Heterotopic pancreas of the jejunum: associations between CT and pathology features. Abdom Imaging 2015;40:38-45. [Crossref] [PubMed]
- Mcheimeche H, Chalaby L, Saheli R, et al. Asymptomatic ectopic pancreas with low grade dysplasia incidentally foundarising from the jejunum during laparoscopic mini gastric bypass: case report. Adv Obes Weight Manag Control 2018;8:185-6.
Cite this article as: Saeed MF, Verhagen KR, Albinali S, Juma IM. A case report and literature review: incidental jejunal ectopic pancreatic tissue in an emergency bowel exploration for suspected intussusception. AME Case Rep 2019;3:24.