
Use of image-guided bone scalpel for resection of spine tumors: technical note
Introduction
Image-guided navigation is a commonly used surgical tool. In oncologic cranial neurosurgery, for instance, it is used for optimizing craniotomy size and placement, defining tumor borders, and localizing important structures to achieve the planned surgical goal while reducing complications (1,2). Given the widespread use of image-guided navigation, it should come as no surprise that more and more surgeons are applying this valuable technology to spinal cases. The most common application in spine surgery has been for stereotactic placement of spinal instrumentation, such as pedicle screws (3,4). Another use for image-guidance is in spinal oncology, where Enneking grade of musculoskeletal tumors often calls for aggressive resection strategies (5). For instance, an Enneking appropriate wide or marginal excision compared to an intralesional excision has a significant effect on tumor recurrence for sacral chordomas and on both survival and tumor recurrence for spinal sarcomas (6,7). Given the importance of Enneking appropriate resection strategies in spinal oncology, tools that could help achieve this goal could prove useful. The following cases involving primary spinal tumor resection illustrate our technique in which an ultrasonic bone scalpel (UBS) was registered to an image-guided navigation system to assist with resection of the tumor.
Case presentation
Case 1
The patient is a 69-year-old male who presented with low back and buttock pain. He was neurologically intact with no notable findings on physical exam. Computed tomography (CT), followed by magnetic resonance imaging (MRI) of his lumbar spine (Figure 1A,B), were obtained, showing a 7.5 cm × 6.0 cm × 6.0 cm lytic lesion in his lower sacrum and coccyx extending anteriorly into the presacral space. A CT-guided biopsy was performed, which was consistent with a chordoma.
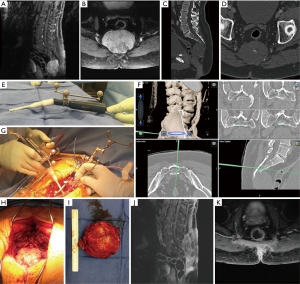
An en bloc resection was planned with expertise from a multidisciplinary team consisting of neurosurgery, surgical oncology (with significant experience treating gastrointestinal malignancies), and plastic and reconstructive surgery. The surgical plan called for a sacrectomy below the level of the S3 foramina with ligation of the bilateral S3 nerves via a modified midline Kraske approach (8). Prior to surgery, the patient underwent a repeat CT scan of the lumbar, sacral, and coccygeal region (Figure 1C,D) with 0.8 mm axial cuts to facilitate accurate image-guided navigation. Intraoperative somatosensory evoked potentials and electromyography were utilized to allow monitoring of the patient’s neurologic status throughout the operation. He was positioned prone on an Andrews table. The incision and dissection spanned from L5 to the distal end of the sacrum and incorporated the prior biopsy tract, which was excised as part of the dissection. The image-guided navigation tracking device was attached to the L5 spinous process and the pre-operative 0.8 mm cut CT scan was registered to the patient using the navigation platform (BrainLAB Curve©, BrainLAB AG, Munich, Germany). Navigation was used to confirm the tumor location below the S3 neural foramina. A midline laminectomy was then performed from S1 to the superior portion of the S3 neural foramina. The thecal sac was ligated just below the S3 nerve roots after neurolysis of sacral nerves. In order to mobilize the sacrum, ligament and fascial attachments were released. The UBS (Sonopet Ultrasonic Aspirator©, Stryker Corporation, MI, USA) was then registered to the navigation platform by attaching a tracking probe clamp to the instrument (Figure 1E). The navigated scalpel was used to ensure all sacral bone cuts began >5 mm outside of the tumor margin and continued both ventrally and laterally at least 5 mm outside the margin as defined on imaging (Figure 1F). The navigated bone scalpel was used to perform the sacral osteotomy >5 mm outside the margins of the chordoma (Figure 1G). The remainder of the case, including a coccygectomy, was then performed in a standard fashion. The total operative duration for the case was 5 hours and 11 minutes.
Pathology confirmed negative margins with no violation of the tumor capsule. Figure 1H and 1I show an intraoperative view of the tumor and the marginally excised specimen, respectively. Post-operatively, the patient experienced mild urinary retention and had mild left foot tingling. He was discharged to acute rehab on post-operative day 8. Surveillance MRI scans at up to 36 months (Figure 1J,K) were negative for evidence of local recurrence. After 3 years’ follow-up, the patient denies any urinary or fecal incontinence, urinary retention, or sexual dysfunction throughout his post-operative recovery.
Case 2
The patient is a 61-year-old female who presented with several weeks of worsening back pain and new onset right lower extremity radicular pain and urinary retention. On exam, she had full strength and normal sensation in both legs. An MRI revealed a homogenously enhancing destructive L4 vertebral body mass with extension of the mass into the inferior endplate of L3 and the superior endplate of L5 (Figure 2A,B). It also exhibited extension into the paravertebral soft tissue and the spinal canal, resulting in significant spinal canal and bilateral neural foramina stenosis. A CT guided biopsy revealed a malignant spindle cell neoplasm. CT scans of her chest, abdomen, and pelvis did not reveal any evidence of malignancy or metastasis. A multidisciplinary team consisting of neurosurgery and surgical oncology proceeded with a staged resection of the tumor, consisting of a posterior resection and instrumentation followed the next day by an anterior resection and instrumentation.
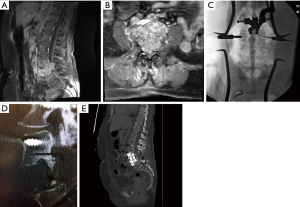
For the first stage of the procedure, a standard dissection from L2 to S1 was performed. Pedicle screws were placed at all levels from L2 to S1, except at L4, using a combination of fluoroscopy and image-guided navigation (BrainLAB Curve©) with the pre-operative 0.8 mm cut CT scan (Figure 2C). Laminectomies were performed from the L3 pedicle to the L5 pedicle, with the L4 pedicles removed bilaterally. Using navigation, a discectomy was completed at L3/4 and L4/5. The UBS (Sonopet Ultrasonic Aspirator©) was then registered to the navigation platform as described in the above case and utilized to perform the bone cuts through the posterior vertebral bodies at L3 and L5, outside of the tumor margins. The navigated UBS was particularly useful for ensuring the vertebral body bone cuts extended anteriorly to the 10 o’clock and 2 o’clock position in order to ensure that the bilateral anterior cuts could be completed from a unilateral access. A sheet of Gore-Tex was then positioned anterior to the thecal sac and L3 and L4 nerve roots. Appropriate rods were placed and secured to the pedicle screws. The following day, an anterior approach was undertaken from the left side, given that the tumor somewhat favored the left side, to complete the tumor resection with an L4 corpectomy and partial L3 corpectomy followed by instrumented fusion. The total operative time of the procedure was 14 hours and 25 minutes (6 hours and 10 minutes for the posterior stage and 8 hours and 15 minutes for the anterior stage). Post-operatively, the patient did not experience any neurologic deficits. The final diagnosis was poorly differentiated sarcomatoid carcinoma. The patient underwent 70 Gray of adjuvant local radiation in 39 equal fractions. No recurrence was noted on 3-month follow up imaging (Figure 2D,E).
Discussion
The ability to apply standard image-guided navigation to spinal procedures was initially limited by an inability to accurately register the patient’s anatomy to pre-operative imaging, as a result of motion between the mobile skin surface and relatively rigid underlying bones (9). In 1996, Foley and Smith published a solution to the limitations of registration by utilizing the dorsal aspect of the spine for registration in conjunction with a dynamic reference array that attaches directly to the spine (10). As the spine curvature varies between pre-operative imaging obtained in a supine position and surgery conducted in a prone position, registration must be done on individual bones to ensure accurate navigation. This limitation can be circumvented if the imaging to be used for navigation is obtained after surgical positioning is completed, for example with an O-arm. Prior studies of navigation assisted pedicle screw placement have demonstrated successful and safe insertion of pedicle screws in all regions of the spine (cervical, thoracic, lumbar, sacral levels) using navigation (11). In one metanalysis which included all regions of the spine and compared 8,539 screws, Shin et al. found increased accuracy of navigated pedicle screw placement, with pedicle breach in only 6% of navigated screws compared to 15% with free hand (12).
The logical progression for applying this tool to spine surgery is to utilize image-guided navigation in surgical spinal oncology to assist in defining tumor margins intraoperatively. There are several manuscripts describing use of stereotactic navigation in spinal tumor resection, including resection of a series of complex cervical spinal tumors, C1 aneurysmal bone cyst, and resection of a sacral Ewing’s sarcoma (13-15). Many other articles have reported using the navigation in spine tumor surgery for implant placement (16-18). Dasenbrock et al. described their experience completing a resection of three sacral chordomas using image-guided navigation with the tracking system registered to a drill for the bony resection and a navigation probe used to confirm margins throughout the case (19). Smitherman et al. published their experience using a navigation-registered osteotome and a navigation probe for performing an en bloc resection of a thoracic spine giant cell tumor (20). Our technique expands on previously studied uses of image-guided navigation and is distinguished from these reports by the use of an UBS registered to the navigation system.
UBS utilize high frequency vibrations causing repetitive impacts at the inelastic bone surface, resulting in cleavage of the bone (21). Soft tissue, with more elastance relative to bone, is better able to absorb impact energy, avoiding inadvertent violation of soft tissue structures (22). UBS systems come equipped with irrigation systems, which provide cooling to the UBS blade tip and minimize thermal damage to the surrounding tissues (21). Prior investigations have reported on the safety of UBS in spinal surgery (23-25). Use of UBS in adult cervical spondylotic myelopathy, adult cervical corpectomy, and adolescent posterior instrumented fusion for idiopathic scoliosis have all been associated with lower blood loss (26-28). Other potential advantages include higher precision, lower rates of durotomies, and decreased operative times (22,26,27). We feel these potential advantages of using an UBS in bone dissection distinguish it from manual osteotomes, which require malleting to achieve bone cuts and are non-hemostatic in their cutting action, and high-speed drills, which are less precise and can be associated with high temperatures if inadequate irrigation is used (29,30).
The authors acknowledge that utilizing image-guided navigation for tumor resection oftentimes will require otherwise unnecessary repeat imaging for navigation protocols. As such, while there is likely decreased intraoperative radiation compared to fluoroscopy, the patient may actually incur more overall radiation from repeat imaging studies and the thin slice protocol required for navigation. In the future, there are several additional modifications to our technique we would like to explore. One nuance would be merging pre-operative MRI with CT imaging to improve tumor definition, as described in the manuscript by D’Andrea et al. (13). Additionally, utilization of a navigated monopolar cautery could prove useful for maintaining margins and avoiding tumor capsule violation when performing soft tissue dissections of these tumors. Future studies examining if using navigation in this manner allows for faster and more accurate implant placement and tumor resection, thus decreasing radiation exposure to staff and operative time for patients, will be important next steps.
Conclusions
Any tool that could potentially optimize a surgeon’s ability to achieve a clinically appropriate resection, should be embraced, given the impact on patient outcomes. We feel that spinal navigation represents such a tool, as described here in two promising cases of successful surgical treatment of spinal tumors. We feel that our experience using an UBS registered to a navigation system has provided an elegant and accurate tool for assisting in the resection of challenging lesions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patients for publication of this case report and any accompanying images.
References
- Barnett GH, Kormos DW, Steiner CP, et al. Use of a frameless, armless stereotactic wand for brain tumor localization with two-dimensional and three-dimensional neuroimaging. Neurosurgery 1993;33:674-8. [PubMed]
- Sipos EP, Tebo SA, Zinreich SJ, et al. In vivo accuracy testing and clinical experience with the ISG Viewing Wand. Neurosurgery 1996;39:194-202; discussion 202-4. [Crossref] [PubMed]
- Tian NF, Huang QS, Zhou P, et al. Pedicle screw insertion accuracy with different assisted methods: a systematic review and meta-analysis of comparative studies. Eur Spine J 2011;20:846-59. [Crossref] [PubMed]
- Mason A, Paulsen R, Babuska JM, et al. The accuracy of pedicle screw placement using intraoperative image guidance systems. J Neurosurg Spine 2014;20:196-203. [Crossref] [PubMed]
- Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 1980.106-20. [PubMed]
- Gokaslan ZL, Zadnik PL, Sciubba DM, et al. Mobile spine chordoma: results of 166 patients from the AOSpine Knowledge Forum Tumor database. J Neurosurg Spine 2016;24:644-51. [Crossref] [PubMed]
- Sciubba DM, De la Garza Ramos R, Goodwin CR, et al. Clinical, surgical, and molecular prognostic factors for survival after spinal sarcoma resection. Neurosurg Focus 2016;41:E9. [Crossref] [PubMed]
- Onaitis M, Ludwig K, Perez-Tamayo A, et al. The Kraske procedure: a critical analysis of a surgical approach for mid-rectal lesions. J Surg Oncol 2006;94:194-202. [Crossref] [PubMed]
- Holly LT, Foley KT. Intraoperative spinal navigation. Spine (Phila Pa 1976) 2003;28:S54-61. [Crossref] [PubMed]
- Foley KT, Smith MM. Image-guided spine surgery. Neurosurg Clin N Am 1996;7:171-86. [Crossref] [PubMed]
- Overley SC, Cho SK, Mehta AI, et al. Navigation and Robotics in Spinal Surgery: Where Are We Now? Neurosurgery 2017;80:S86-99. [Crossref] [PubMed]
- Shin BJ, James AR, Njoku IU, et al. Pedicle screw navigation: a systematic review and meta-analysis of perforation risk for computer-navigated versus freehand insertion. J Neurosurg Spine 2012;17:113-22. [Crossref] [PubMed]
- D'Andrea K, Dreyer J, Fahim DK. Utility of Preoperative Magnetic Resonance Imaging Coregistered with Intraoperative Computed Tomographic Scan for the Resection of Complex Tumors of the Spine. World Neurosurg 2015;84:1804-15. [Crossref] [PubMed]
- Neva J, Smith BW, Joseph JR, et al. Use of intraoperative navigation for reconstruction of the C1 lateral mass after resection of aneurysmal bone cyst. World Neurosurg 2017;102:693.e21-7. [Crossref] [PubMed]
- Al Eissa S, Al-Habib AF, Jahangiri FR. Computer-Assisted Navigation During an Anterior-Posterior En Bloc Resection of a Sacral Tumor. Cureus 2015;7:e373. [PubMed]
- Hussain I, Navarro-Ramirez R, Lang G, et al. 3D Navigation-guided Resection of Giant Ventral Cervical Intradural Schwannoma With 360-Degree Stabilization. Clin Spine Surg 2018;31:E257-65. [Crossref] [PubMed]
- Bandiera S, Ghermandi R, Gasbarrini A, et al. Navigation-assisted surgery for tumors of the spine. Eur Spine J 2013;22 Suppl 6:S919-24. [Crossref] [PubMed]
- Guppy KH, Chakrabarti I, Banerjee A. The use of intraoperative navigation for complex upper cervical spine surgery. Neurosurg Focus 2014;36:E5. [Crossref] [PubMed]
- Dasenbrock HH, Clarke MJ, Bydon A, et al. En bloc resection of sacral chordomas aided by frameless stereotactic image guidance: a technical note. Neurosurgery 2012;70:82-7; discussion 87-8. [PubMed]
- Smitherman SM, Tatsui CE, Rao G, et al. Image-guided multilevel vertebral osteotomies for en bloc resection of giant cell tumor of the thoracic spine: case report and description of operative technique. Eur Spine J 2010;19:1021-8. [Crossref] [PubMed]
- Tarazi N, Munigangaiah S, Jadaan M, et al. Comparison of thermal spread with the use of an ultrasonic osteotomy device: Sonopet ultrasonic aspirator versus misonix bonescalpel in spinal surgery. J Craniovertebr Junction Spine 2018;9:68-72. [PubMed]
- Sanborn MR, Balzer J, Gerszten PC, et al. Safety and efficacy of a novel ultrasonic osteotome device in an ovine model. J Clin Neurosci 2011;18:1528-33. [Crossref] [PubMed]
- Hu X, Ohnmeiss DD, Lieberman IH. Use of an ultrasonic osteotome device in spine surgery: experience from the first 128 patients. Eur Spine J 2013;22:2845-9. [Crossref] [PubMed]
- Al-Mahfoudh R, Qattan E, Ellenbogen JR, et al. Applications of the ultrasonic bone cutter in spinal surgery--our preliminary experience. Br J Neurosurg 2014;28:56-60. [Crossref] [PubMed]
- Bydon M, Xu R, Papademetriou K, et al. Safety of spinal decompression using an ultrasonic bone curette compared with a high-speed drill: outcomes in 337 patients. J Neurosurg Spine 2013;18:627-33. [Crossref] [PubMed]
- Onen MR, Yuvruk E, Akay S, et al. The Reliability of the Ultrasonic Bone Scalpel in Cervical Spondylotic Myelopathy: A Comparative Study of 46 Patients. World Neurosurg 2015;84:1962-7. [Crossref] [PubMed]
- Dave BR, Degulmadi D, Dahibhate S, et al. Ultrasonic bone scalpel: utility in cervical corpectomy. A technical note. Eur Spine J 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Bartley CE, Bastrom TP, Newton PO. Blood Loss Reduction During Surgical Correction of Adolescent Idiopathic Scoliosis Utilizing an Ultrasonic Bone Scalpel. Spine Deform 2014;2:285-90. [Crossref] [PubMed]
- Hosono N, Miwa T, Mukai Y, et al. Potential risk of thermal damage to cervical nerve roots by a high-speed drill. J Bone Joint Surg Br 2009;91:1541-4. [Crossref] [PubMed]
- Matthes M, Pillich DT, El Refaee E, et al. Heat Generation During Bony Decompression of Lumbar Spinal Stenosis Using a High-Speed Diamond Drill with or without Automated Irrigation and an Ultrasonic Bone-Cutting Knife: A Single-Blinded Prospective Randomized Controlled Study. World Neurosurg 2018;111:e72-81. [Crossref] [PubMed]
Cite this article as: Towner JE, Piper KF, Schoeniger LO, Qureshi SH, Li YM. Use of image-guided bone scalpel for resection of spine tumors: technical note. AME Case Rep 2018;2:48.


