Clear cell “sugar” tumor of the lung: a case report and review of the literature
Introduction
Clear cell “sugar” tumor of the lung (CCTL) is a benign lesion in the lung that was first described in 1963 by Liebow and Castleman in four cases. In 1971, they presented twelve more cases where they determined that the tumor resembled renal cell carcinoma (RCC) as it has thin cell walls, high levels of glycogen and no evidence of necrosis (1). Common tumor markers found in CCTL include S100 and HMB45 which can differentiate it from RCC (2). CCTL most commonly occurs in adults during the fourth to sixth decade of life but has been reported in children as young as eight (2). Typically, it is an incidental finding (2).
Case presentation
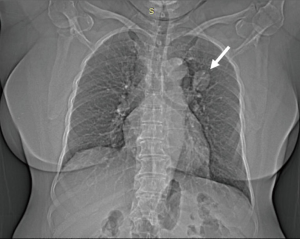
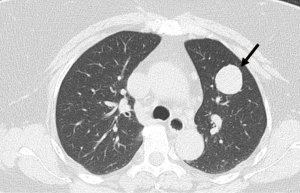
A 61-year-old female, with a significant past medical history of hypertension and hyperlipidemia, presented to our institution with an upper respiratory tract infection. A chest X-ray was completed which showed a suspicious left lung mass (Figure 1). The patient denied any coughs, hemoptysis, or shortness of breath. She denied any smoking history or family history of cancer. A computed tomography (CT) scan of the chest was performed which demonstrated a single spiculated 3.0×2.7 cm mass in the left upper lobe (Figure 2).
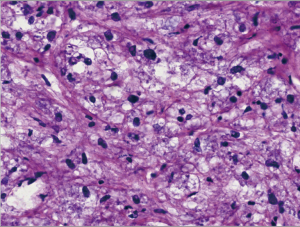
A diagnostic bronchoscopy was performed with fine needle biopsy of the mass. Histology of the tumor showed cytoplasmic periodic acid-Schiff (PAS) positive clear cells without discernible mitotic activity, necrosis or significant atypia, which suggested CCTL. The immunohistochemistry was positive for HMB45/MART-1 and Vimentin. Additionally, the staining was negative for pan-cytokeratin, CAM5.2, SOX10, Thyroid transcription factor-1 or Desmin.
The patient subsequently underwent a video-assisted thoracoscopic surgery wedge resection of the tumor. Pathology confirmed the diagnosis of a clear cell tumor of the lung (Figure 3). The tumor measured 3×2.5×2.5 cm and stained positive for HMB-45 and CD34. It was negative for S-100, AE1/3, SMA, Calponin, GFAP, Desmin, TTF-1, P40 and PAX-8. The patient did well postoperatively and was discharged home on postoperative day 2.
Discussion
There have been numerous hypotheses discussing the origin of CCTL. Becker and Soifer in 1971 noted the similarities between the appearance of the liver in Pompe’s disease (type II glycogen storage disease) and CCTL. This suggests that CCTL may be a result of a similar but isolated metabolic dysfunction in the lung involving abnormal lysosomes (3). Additionally, they demonstrated the presence of neurosecretory granules in CCTL making it a variant of the pulmonary tumors that originate from Kulchitsky cells (3).
Bonetti et al. in 1994 proposed that CCTL originated from perivascular epithelioid cells because of its similarities to lymphangioleiomyomatosis (LAM) and angiomyolipoma, which are associated with tuberous sclerosis (TSC). Therefore, they hypothesized that CCTL may be found in patients with TSC (4). Later, this hypothesis was confirmed by Flieder et al. in 1997 as they reported the first case of CCTL in a patient with TSC (5). They suggested that CCTL be added as a pulmonary manifestation of TSC (5). However, CCTL has also been reported as occurring in the presence of LAM without the other features of TSC (6).
CCTL may also be related to tumors known as primary extrapulmonary sugar tumors (PESTs). Tazelaar et al. in 2001 found tumors with glycogen rich clear cells and HMB-45 positivity in the low rectum, vulva, cardiac interatrial septum, and rectum (7).
CCTL can present with coughing, shortness of breath or hemoptysis but is most often an incidental finding of a coin lesion or solitary pulmonary nodule on radiographic imaging (8-10). Some unique presentations found in the literature include thrombocytosis (platelet count >1,000,000/mm3), which resolved after resection of the CCTL (11,12). CCTL has also been found concurrently with minute pulmonary meningothelial-like nodules in the presence of invasive rectal adenocarcinoma (13). The two benign lung lesions mimicked metastasis, therefore, the authors emphasized the importance of an awareness of such tumors to correctly stage and provide the appropriate treatment for patients with malignancies (13). Additionally, in another case, RCC was found 20 months after the CCTL was resected (14). The authors concluded that the primary renal lesion presented after an instance of solitary metastasis (14).
The primary management of CCTL is complete resection of the affected lobe (lobectomy). This is typically sufficient and chemotherapy is not normally required. Few cases of malignant CCTL have been reported in the literature, one of which presented with a two-month history of a sensation of chest suppression (15). Therefore, it is imperative to schedule follow-ups with patients to rule out metastasis. It is also important to maintain surveillance for RCC and should be ruled out immediately as both tumors are similar on histology. Surveillance for RCC should be maintained indefinitely.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Liebow AA, Castleman B. Benign clear cell (“sugar”) tumors of the lung. Yale J Biol Med 1971;43:213-22. [PubMed]
- Dail DH, Hammar SP, Colby TV. Uncommon Tumors. In: Pulmonary Pathology—Tumors. New York: Springer Science & Business, Media 2012:219-24.
- Becker NH, Soifer I. Benign clear cell tumor (“sugar tumor”) of the lung. Cancer 1971;27:712-9. [Crossref] [PubMed]
- Bonetti F, Pea M, Martignoni G, et al. Clear cell (“sugar”) tumor of the lung is a lesion strictly related to angiomyolipoma--the concept of a family of lesions characterized by the presence of the perivascular epithelioid cells (PEC). Pathology 1994;26:230-6. [Crossref] [PubMed]
- Flieder DB, Travis WD. Clear cell “sugar” tumor of the lung: association with lymphangioleiomyomatosis and multifocal micronodular pneumocyte hyperplasia in a patient with tuberous sclerosis. Am J Surg Pathol 1997;21:1242-7. [Crossref] [PubMed]
- Hironaka M, Fukayama M. Regional proliferation of HMB-45-positive clear cells of the lung with lymphangioleiomyomatosislike distribution, replacing the lobes with multiple cysts and a nodule. Am J Surg Pathol 1999;23:1288-93. [Crossref] [PubMed]
- Tazelaar HD, Batts KP, Srigley JR. Primary extrapulmonary sugar tumor (PEST): a report of four cases. Mod Pathol 2001;14:615-22. [Crossref] [PubMed]
- Küng M, Landa JF, Lubin J. Benign clear cell tumor (“sugar tumor”) of the trachea. Cancer 1984;54:517-9. [Crossref] [PubMed]
- Santana AN, Nunes FS, Ho N, et al. A rare cause of hemoptysis: benign sugar (clear) cell tumor of the lung. Eur J Cardiothorac Surg 2004;25:652-4. [Crossref] [PubMed]
- Kavunkal AM, Pandiyan MS, Philip MA, et al. Large clear cell tumor of the lung mimicking malignant behavior. Ann Thorac Surg 2007;83:310-2. [Crossref] [PubMed]
- Sen S, Senturk E, Kuman NK, et al. PEComa (clear cell “sugar” tumor) of the lung: a benign tumor that presented with trombocytosis. Ann Thorac Surg 2009;88:2013-5. [Crossref] [PubMed]
- Yazak V, Sargin G, Yavasoglu I, et al. Lung Clear “Sugar” Cell Tumor and JAK V617F Positive Essential Thrombocythemia: a Simple Coincidence? Mediterr J Hematol Infect Dis 2013;5. [Crossref] [PubMed]
- Arafah MA, Raddaoui E, Alsheikh A, et al. Mimicry of sugar tumor and minute pulmonary meningothelial-like nodule to metastatic lung deposits in a patient with rectal adenocarcinoma. Ann Saudi Med 2013;33:400-3. [Crossref] [PubMed]
- Wills JS, Hewes AC. Benign clear cell tumor of the lung: a cautionary tale. Urol Radiol 1980;2:255-7. [Crossref] [PubMed]
- Ye T, Chen H, Hu H, Wang J, Shen L. Malignant clear cell sugar tumor of the lung: patient case report. J Clin Oncol 2010;28:e626-8. [Crossref] [PubMed]
Cite this article as: Chang M, Lim D, Genovesi M. Clear cell “sugar” tumor of the lung: a case report and review of the literature. AME Case Rep 2018;2:40.