Rare isolated synchronous splenic metastasis in a patient with type II papillary renal cell carcinoma
Introduction
Metastatic disease is common in renal cell carcinoma (RCC) with a third of cases being synchronous. RCC is known to metastasize to any organ in the body but isolated splenic metastasis is extremely rare. Here we report a case of synchronous splenic metastasis from type II papillary RCC with 80% sarcomatoid change.
Case presentation
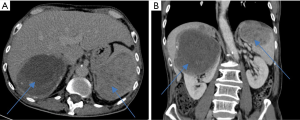
A 75-year-old Chinese man was referred to the General Surgery department with an incidental finding of anaemia. He did not have any significant past medical history of note. He was an ex-smoker and non-alcoholic. He underwent an oesophagogastroduodenoscopy which revealed mild oesophagitis and non-contrast computed tomography (CT) colonography which revealed diverticular disease and a 10.4 cm × 9.7 cm × 7.8 cm right renal mass (Figure 1A) with a subcentimetre indeterminate splenic hypodensity (Figure 1B). He was however lost to follow-up and only presented to urology 9 months later with lower urinary tract symptoms with microscopic haematuria. He reported a loss of weight of 8 kilograms over 1 year.
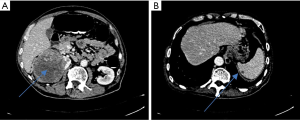
On physical examination, there was a ballotable right kidney. A CT of his abdomen and pelvis revealed an 11.2 cm × 10.1 cm × 9.6 cm right enhancing, heterogenous renal mass with infiltration into the ipsilateral adrenal gland and into segment 7 of the liver (Figure 2). There was no renal vein tumour thrombus. The splenic lesion was now 9.9 cm × 7.6 cm × 4.8 cm which was suspicious for metastasis (Figure 2). A CT of his thorax and bone scan did not reveal other metastases. His serum chemistry is as follows: white blood cells (WBC)—5.7, haemoglobin—9.1, platelets—405, absolute neutrophil count—3.00, sodium (Na)—130, potassium (K)—4.4, creatinine (Cr)—73, urea (Ur)—3.6, calcium (Ca)—2.31, albumin (Alb)—27, liver function tests (LFT)—normal except for alkaline phosphatase (ALP) 216. This was a good prognosis risk group based on the International Metastatic Renal-Cell Carcinoma Database Consortium (IMDC) prognostic criteria (1). Pre-operatively, he was given triple vaccination (meningococcal, pneumococcal, and streptococcal). He underwent right radical nephrectomy and splenectomy and en bloc right liver wedge resection.
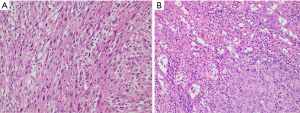
Post-operatively, his stay was complicated by a right sided pleural effusion and collection at the splenic bed which were drained. He was discharged on post-operative day (POD) 20 and the splenic bed drain was removed in the clinic on POD 25 in the outpatient setting. Histology revealed type II papillary RCC with 80% sarcomatoid changes in both the kidney and spleen (Figure 3). The tumour was invading into the adrenal gland (pT4) but not involving the liver. Margins were negative.
Six cycles of adjuvant chemotherapy (gemcitabine) was administered subsequently. However, the patient developed disease recurrence in the spine at the 6th post-operative month with a pathological fracture at T3 and cord indentation, for which he underwent posterior decompression and instrumentation and palliative radiotherapy. He recovered well and remains ambulant. Surveillance scans at 16 months post right radical nephrectomy and splenectomy showed no tumour recurrence or new metastasis.
Discussion
Worldwide each year, there are over 270,000 new cases of RCC diagnosed and more than 100,000 deaths from RCC; RCC represents 2–3% of all cancers (2).
RCC is usually asymptomatic. The prevalence of the classic RCC triad of flank pain, gross hematuria and a palpable abdominal mass is rare and paraneoplastic syndromes such as hypercalcemia, hypertension, polycythemia and Stauffer’s syndrome occur in 20% of patients. A small number of patients present with symptoms caused by metastatic RCC (3). RCC is known to metastasize to every known organ but the most common sites are the lung, bone, liver, adrenal gland, brain and contralateral kidney. Splenic metastasis occurs in 2.3–7.1% of cancer cases, of which 95% are carcinomas (4). Isolated splenic metastases from RCC are extremely rare with only about nine cases being reported in the literature (5). The possible reasons that splenic metastases in nonlymphoid tumours are rare include: the sharp angle at the origin of the splenic artery, the rhythmic contraction of the splenic sinusoids, the scarcity of lymphatic vessels extending into the parenchyma, the protective role of the splenic capsule and the microenvironment that inhibits the growth of cancer cells (6).
In this case, the rapid growth of the splenic metastases from type II papillary RCC with high percentage of sarcomatoid change is illustrated. In 9 months, a subcentimetre splenic hypodensity grew to 9.9 cm. This is consistent with the rapid growth rates of RCC metastases as depicted in other case reports (7). In contrary, the reported median linear growth rate of small renal mass (≤4 cm) was 0.28 cm/year and median volumetric growth rate was 0.75 cm3/year and median volume doubling time was 1.41 years (8). Hence, it is important for the clinician to adequately stage RCC patients and avoid any delays in biopsies/treatment of suspicious lesions. If the disease is metastatic, treatment is usually palliative and the prognosis is guarded.
The best treatment for isolated splenic metastases in any type of cancer is still debatable given the rarity of it. In a recent meta-analysis and systemic review, Zaid et al. demonstrated that complete metastasectomy for patients with metastatic RCC is associated with improved survival compared with no or incomplete metastasectomy (9). In our case, right radical nephrectomy with en bloc right liver resection and splenectomy were performed with negative margins to achieve removal of the primary tumour and complete metastasectomy. In view of the high percentage of sarcomatoid change (80%), the oncologists have offered him adjuvant chemotherapy and the patient will be on close surveillance for follow-up.
Acknowledgements
The authors would like to express their thanks to Dr. Wong Chin Fong from the Department of Pathology, Tan Tock Seng Hospital, Singapore, for the histology slides used in this case report.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Verbal informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Ko JJ, Xie W, Kroeger N, et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol 2015;16:293-300. [Crossref] [PubMed]
- Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol 2011;60:615-21. [Crossref] [PubMed]
- Kavoussi L, Novick A, Partin A, et al. editors. Campbell-Walsh Urology. 10th ed. Philadelphia, PA: Elsevier Saunders, 2012.
- Compérat E, Bardier-Dupas A, Camparo P, et al. Splenic metastases: clinicopathologic presentation, differential diagnosis, and pathogenesis. Arch Pathol Lab Med 2007;131:965-9. [PubMed]
- Zhang L, Pasquale D, Le M, et al. Isolated splenic metastasis in a patient with two distinct genitourinary malignancies. J Community Support Oncol 2015;13:229-30. [Crossref] [PubMed]
- Abi Saad GS, Hussein M, El-Saghir NS, et al. Isolated splenic metastasis from colorectal cancer. Int J Clin Oncol 2011;16:306-13. [Crossref] [PubMed]
- Selvi F, Faquin WC, Michaelson MD, et al. Three Synchronous Atypical Metastases of Clear Cell Renal Carcinoma to the Maxillary Gingiva, Scalp and the Distal Phalanx of the Fifth Digit: A Case Report. J Oral Maxillofac Surg 2016;74:1286.e1-9. [Crossref] [PubMed]
- Lee SW, Sung HH, Jeon HG, et al. Size and Volumetric Growth Kinetics of Renal Masses in Patients With Renal Cell Carcinoma. Urology 2016;90:119-24. [Crossref] [PubMed]
- Zaid HB, Parker WP, Safdar NS, et al. Outcomes following complete surgical metastasectomy for patients with metastatic renal cell carcinoma: a systematic review and meta-analysis. J Urol 2017;197:44-9. [Crossref] [PubMed]
Cite this article as: Liu Z, Chow MW, Lua AH, Tan RB. Rare isolated synchronous splenic metastasis in a patient with type II papillary renal cell carcinoma. AME Case Rep 2018;2:9.


