Hump-like giant desmoid tumor of the chest: a postresectional reconstruction challenge
Introduction
Desmoid tumors (DT) are rare soft tissue neoplasms with uncertain etiology accounting for 0.03% of all tumors. Due to their high tendency to recur even after radical surgical resection, they have been classified as low grade fibrosarcomas (1). Nearly half of DT arises in the abdominal region although extra-abdominal sites including chest wall, shoulder girdle, inguinal region, head and neck have been also reported (2). Chest wall localization is uncommon having being reported in 10% to 20% of cases (3). Usually chest wall DT has various sizes ranging from 5 to 10 cm and rarely reaches dimensions larger than 20 cm (4). The presentation of DT is usually of a painless palpable mass (5). Sometimes this mass may compress the underlying structures causing pain and can also causes sensory and motor symptoms (5). Definitive diagnosis requires histopathological examination. Computed tomography as well as magnetic resonance imaging can help identify the size and site of the tumor, the invasion of neighboring structures and may prove useful for the radiological follow-up after surgical treatment (6).
In few instances, giant DT can challenge the decision for resection and particularly the optimal way to cover the resulting chest wall defect. We report on a patient with a giant hump-like, recurrent DT of the posterior chest wall, which was surgically resected following subcutaneous placement of multiple silicon tissue expanders to gain redundant skin, which eventually allowed in conjunction with two transposition, cutaneous-adipose flaps, harvested from the upper gluteal region, an optimal and relatively simple reconstruction of the large postresectional defect.
Case presentation
A 33-year-old man with a past history of recurrent DT of the posterior chest wall was referred for redo surgical resection. The patient already underwent previous tumor resection in 2010, 2011 and 2012. After in-hospital admission, at physical examination, the patient disclosed a huge hump-like tumor bulging in the lower third of the posterior chest wall. The patient did not complain of pain despite the large tumor size, which however impaired severely his quality of life and daily activity. Chest computed tomography showed a large tumor involving the soft tissue including prevertebral and paravertebral muscles and bulging posteriorly (Figure 1A). At multidisciplinary evaluation including thoracic and plastic surgeons it was decided to resect the tumor following insertion of three silicone skin expanders (700 cc max volume tissue expanders, two rectangular and one crescent) one (the crescent one) above the tumor and two (the rectangular ones) laterally on each side of the mass. The tissue expanders were inserted in the subcutaneous tissue surrounding the lesion through a blunt dissection, and gradually inflated with saline for approximately 40 days through a valve system (approximately 80–100 cc every 7–10 days) to obtain enough soft tissue to expand the surrounding skin and to allow for the final covering of the large postresectional tissue defect (Figure 1B). Thirty days later, one of the tissue expanders was removed due to local infection and antibiotic therapy was administered to the patient.
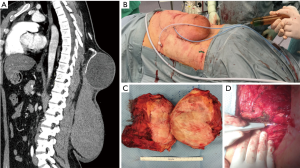
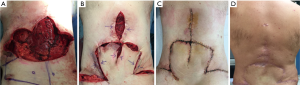
During the operation in October 2016, a “lotus-like” horizontal skin incision was performed and the tumor was progressively excised with macroscopically free margins of at least 3 cm in all directions including the overlying skin. Particular care was devoted to remove all aponeurotic and muscle tissues that might have been potentially infiltrated by the tumor within the deepest plane. After removal of the mass, which was 25 cm × 25 cm in maximal diameters and weighted 3.8 kg (Figure 1C), we employed the argon beam coagulator over the surgical bed in an attempt of destroying macroscopically undetectable local residual tumor cells (Figure 1D). At end of the procedure, the paravertebral muscles were relocated over the spine and three Redon drains were inserted. Finally, two transposition, well vascularized cutaneous-adipose flaps, harvested from the upper gluteal region, were rotated upwards to cover the central 2/3 of the defect, whereas redundant skin tissue obtained laterally following inflation of the expanders was used to cover the lateral and superior part of the defect. Atraumatic staples were used to close the skin. The patient remained well and free of recurrence at last follow-up, 12 months after surgery (Figure 2A-D).
Discussion
DT are usually located within fascial or musculoaponeurotic structures. They disclose firm or rubbery consistency with a tendency to infiltrate surrounding structures (7).
Despite etiology of DT is still unknown, a history of trauma is reported in up to 25% of patients (7). There can be also an association with familial adenomatous polyposis and Gardner’s syndrome as well as a correlation with estrogen blood level (8).
Histopathological examination through a biopsy is mandatory for definitive diagnosis. To prevent recurrence, wide radical resection with negative resection margins is essential although it might prove difficult owing to tumor microscopic invasion of surrounding vascular and neural structures. Recurrence occurs in 25% up to 75% of cases with chest wall involvement independent by the radicality of resection (7) leading some authors to recommend adjuvant therapy including radiation or anti-estrogen therapy (9).
Our case entails a multi-recurrent DT involving soft tissue of lower third of the posterior chest wall, which reached giant dimensions leading to severe impairment of daily-life activity of the patient as well as severe psychological disturbances. At surgical resection, following removal of the mass, which included a large surface of the overlying skin tissue, we added use of the argon beam electrocoagulator onto the surgical bed in an attempt to eliminate residual microscopic foci of neoplastic cells. In fact, argon beam has been recommended for this purpose during surgical resection of malignant pleural mesothelioma (10) but we considered it potentially useful also in our patient because of the high rate of local recurrence of DT (11).
The most challenging surgical problem in our case was related to the choice of an optimal method to cover the wide post-resectional soft tissue defect (35 cm diameter) (Figure 2A). In these instances, rotated muscle flaps including use of latissimus dorsi, deep inferior epigastric perforator, the tensor fascia lata and even free myocutaneous flaps with microvascular anastomosis are all available alternatives, which however prove more technically demanding (12,13). We reasoned however that use of skin expanders might offer a less disfiguring and not functionally impairing option when compared with muscle flaps (14) as demonstrated also by the final cosmetic results in our patient (Figure 2D), which was judged highly satisfactory. In fact, large amounts of newly generated skin tissue can be gained by this method. The biological mechanism underlying this effect is thought to be related not merely to tissue stretching but also to tissue regeneration triggered by a cascade of biochemical pathways inducing cell proliferation (15).
On the other hand, potential complications of tissue expanders include: infections, which occurred in one tissue expander in our case without compromising the final result; exposure of the expander balloon, deflation of the balloon and ischaemic necrosis of the overlying skin (16).
Conclusions
In conclusion, we report on a case of giant hump-like DT, which was radically resected following placement of multiple tissue expanders, which allowed us to satisfactorily cover the resulting large postresectional soft tissue defect. We hypothesize that this simple method, which to the best of our knowledge was employed for the first time for chest wall reconstruction in a site other than breast (13) and sternum (17), might be considered in other similar circumstances as a valid alternative to more complex muscle flap repairs.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Lewis JJ, Boland PJ, Leung DH, et al. The enigma of desmoid tumors. Ann Surg 1999;229:866-72; discussion 872-3. [Crossref] [PubMed]
- Allen PJ, Shriver CD. Desmoid tumors of the chest wall. Semin Thorac Cardiovasc Surg 1999;11:264-9. [Crossref] [PubMed]
- Ibrahim M, Sandogji H, Allam A. Huge intrathoracic desmoid tumor. Ann Thorac Med 2009;4:146-8. [Crossref] [PubMed]
- Kabiri EH, Al Aziz S, El Maslout A, et al. Desmoid tumors of the chest wall. Eur J Cardiothorac Surg 2001;19:580-3. [Crossref] [PubMed]
- Dashiell TG, Payne WS, Hepper NG, et al. Desmoid tumors of the chest wall. Chest 1978;74:157-62. [Crossref] [PubMed]
- Bernard J, Le Breton C, Piriou P, et al. Value of MRI to evaluate extra-abdominal desmoid fibromatosis. J Radiol 2002;83:711-6. [PubMed]
- Shields CJ, Winter DC, Kirwan WO, et al. Desmoid tumours. Eur J Surg Oncol 2001;27:701-6. [Crossref] [PubMed]
- Wilcken N, Tattersall MH. Endocrine therapy for desmoid tumors. Cancer 1991;68:1384-8. [Crossref] [PubMed]
- Meyerson SL, D'Amico TA. Intrathoracic desmoid tumor: brief report and review of literature. J Thorac Oncol 2008;3:656-9. [Crossref] [PubMed]
- Tilleman TR, Richards WG, Zellos L, et al. Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: a phase II prospective study. J Thorac Cardiovasc Surg 2009;138:405-11. [Crossref] [PubMed]
- Rusch VW, Schmidt R, Shoji Y, et al. Use of the argon beam electrocoagulator for performing pulmonary wedge resections. Ann Thorac Surg 1990;49:287-91. [Crossref] [PubMed]
- Bosc R, Lepage C, Hamou C, et al. Management of chest wall reconstruction after resection for cancer: a retrospective study of 22 consecutive patients. Ann Plast Surg 2011;67:263-8. [Crossref] [PubMed]
- Rifaat MA, Amin AA, Bassiouny M, et al. The extended latissimus dorsi flap option in autologous breast reconstruction: A report of 14 cases and review of the literature. Indian J Plast Surg 2008;41:24-33. [Crossref] [PubMed]
- Bodin F, Dissaux C, Steib JP, et al. Complex posterior thoracic wall reconstruction using a crossover combined latissimus dorsi and serratus anterior free flap. Eur J Cardiothorac Surg 2016;49:1008-9. [Crossref] [PubMed]
- De Filippo RE, Atala A. Stretch and growth: the molecular and physiologic influences of tissue expansion. Plast Reconstr Surg 2002;109:2450-62. [Crossref] [PubMed]
- Masser MR. Tissue expansion: a reconstructive revolution or a cornucopia of complications? Br J Plast Surg 1990;43:344-8. [Crossref] [PubMed]
- Park KU, Moquin K. Novel Use of External Tissue Expander for Management of Sternal Wound Dehiscence. Ann Thorac Surg 2015;100:e81-3. [Crossref] [PubMed]
Cite this article as: Elkhouly AG, Cervelli V, Sanese G, Pompeo E. Hump-like giant desmoid tumor of the chest: a postresectional reconstruction challenge. AME Case Rep 2017;1:6.


