Recurrent giant phyllodes tumor of the breast: a case report
Highlight box
Key findings
• Current recommendations for phyllodes tumor (PT) treatment advising wide excision regardless of tumor size should be revised.
What is known and what is new?
• PTs account for 0.3% to 1.0% of all breast tumors. PTs are well known for local recurrence and progression. Less than 10% of these tumors grow larger than 10 cm.
• Although most surgeons would be uncomfortable with not re-excising a borderline PT with positive margins, it would be reasonable to assume a ‘watchful waiting’ strategy for benign lesions.
What is the implication, and what should change now?
• Close clinical and radiologic follow-up may provide a better course of management rather than re-excision when managing positive margins in benign and borderline PTs.
Introduction
Background
Phyllodes tumors (PTs) are rare fibroepithelial tumors. They account for 0.3–1.0% of all breast tumors and often occur in women 35 to 55 years old (1). Although PTs are similar to fibroadenomas (FA), their rapid growth rate, large size, and microscopical findings of stromal hyperplasia and atypia should be suspected.
Rationale and knowledge gap
The World Health Organization (WHO) has issued guidelines to classify PTs as benign, borderline or malignant based on histopathological characteristics of stromal hypercellularity, cytological atypia, mitotic rate, boundary type, stromal overgrowth, and heterostromal differentiation (2). Overall, the majority of PTs are benign, benign tumor occurrence between 35–78% of all PTs, borderline 6–35%, and malignant 14–35% (3). However, the behavior of PTs can vary greatly between different subtypes. In particular, borderline and malignant subtypes exhibit the properties of recurrence and metastasis (4). PTs tend to recur after resection, with increased local recurrence rates in benign (8%), borderline (13%), and malignant (18%) groups (5). Because of the potential for local recurrence and metastasis of PTs, surgery with clear margins remains the primary treatment. The National Comprehensive Cancer Network (NCCN) guidelines recommend extensive resection with a margin of ≥1 cm for PTs (2,6), which is much larger than what is required for breast cancer.
Objective
Recent study has shown that a narrower margin is not associated with an increased risk of recurrence. A 1 mm margin in benign PTs has been advocated (7). Because a tumor positive margin is associated with recurrence, there is a strong preference for re-excision to obtain a negative margin for the tumor. However, little is known whether tumor negative margins are required for all subtypes.
Here in, we present a case of recurrent giant PT without any metastasis that was successfully treated by total mastectomy of left breast in our hospital. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-84/rc).
Case presentation
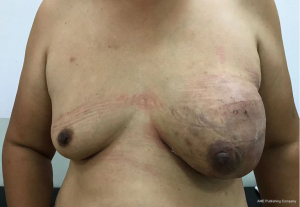
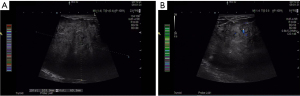
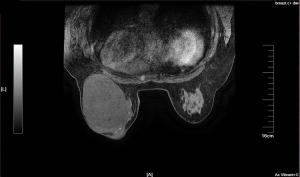
A 49-year-old woman visited the outpatient department in October 2020, who found a left breast palpable tumor for 16 months, with rapid tumor growth in recent months. She had a bilateral breast lumpectomy (outer lower quadrant of left breast, measured 5.4 cm × 4.4 cm; upper outer quadrant of right breast, measured 2.2 cm × 1.5 cm) under local anesthesia in January 2014 and a giant left breast tumor excision (outer lower quadrant of left breast, measured 10.4 cm × 5.5 cm) under local anesthesia in December 2018. Intraoperative frozen pathology showed fibroadenoma of both breasts, postoperative pathology was benign PT without margin status in January 2014. And intraoperative frozen pathology showed left breast fibroepithelial tumor, the postoperative pathology was left breast benign PT without margin evaluation in December 2018. She had no family history of breast disease. Otherwise, there were no systemic symptoms such as fever, fatigue, or weight loss. She noticed the lump 16 months ago, and it began to grow significantly two months ago. Accompanied by redness of the skin and swelling of left breast. There was no nipple discharge and no other masses noticed in the right breast. Physical examination revealed longitudinal scarring on the lateral side of the left areola associated with a previously removed giant left benign PT. Since the tumor occupied most of the breast, the left breast was significantly enlarged and the skin over the tumor was discolored. The mass can move freely through the chest. The skin covering the tumor was weakened, the subcutaneous veins were significantly dilated, and there was no nipple discharge (Figure 1). On palpation, a hard elastic mass 15 cm in diameter with relatively well-delimited, polylobate tumor was palpated of the outernal of the left breast, with incomplete skin adhesion. The right breast examination was unremarkable. No palpable axillary and supraclavicular lymph nodes were found. Laboratory data showed all within normal limits. A chest computerized tomography (CT) scan and abdomen ultrasound (US) showed no evidence of distant metastasis. Breast US and breast magnetic resonance imaging (MRI) showed normal right breast and left breast lesion. US examination showed a large, oval, circumscribed, hypoechoic left breast mass, with vascular flow in the periphery, and areas of posterior acoustic enhancement, corresponding to the palpable mass reported by the patient—a Breast Imaging Reporting and Data System (BI-RADS) 5 lesion (Figure 2). Measurements at that time were greater than 10.5 cm × 7.0 cm, and hypoechoic mass with smooth, well-defined margins. MRI revealed a huge lobulated mass in the left breast, approximately 12.8 cm × 9.1 cm × 11.6 cm in size, with several axillary lymph nodes enlargement (Figure 3). Core needle biopsy (CNB) histological examination showed fibroepithelial tumor with cell proliferation. Differential diagnosis included FA and PTs.
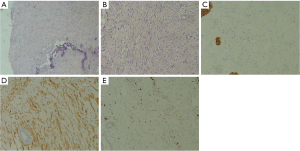
Considering the patient’s age and tolerability, a left mastectomy was performed after thorough discussion with the patient. The patient tolerated the surgery well and without any complications. Macroscopically, the tumor was 10.0 cm in diameter, grayish white, encapsulated, and without necrosis. Histopathology shows that the tumor is lobulated, with fibroadenomatoid changes showing prominent stromal cellularity, pseudoangioma hyperplasia (Figure 4A). There is mild to moderate cell heteromorphism and 5/10 high power fields (HPFs) mitotic activity, with domain obscure boundary and infiltrate adipose tissue (Figure 4B). The surgical margin was not involved by the tumor. A spindle cell lesion that was negative for cytokeratin (CK) (Figure 4C) and positive for cluster of differentiation 34 (CD34) (Figure 4D), Ki-67 (+5%) (Figure 4E). Since CK staining was negative, metaplastic carcinoma can be excluded. Then the tumor was diagnosed as being a borderline PT.
During the first 24 months of follow-up, there was no evidence of local or distant recurrence in clinical, breast US exams, mammographic, and CT scans at each follow-up.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee (s) and with the Helsinki Declaration (as revised in 2013). Ethical approval was granted by the hospital’s ethics committee (No. YN2023-038-01). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Key findings
PTs are rare and comprise 0.3–1% of all breast tumors and 2–3% of breast fibroepithelial tumors. Generally, PTs are most common in patients during the fourth or fifth decade of life, older than FA, which is most common in 20 years old. In 2003, the WHO named it PT and categorized it into benign, borderline and malignant subtypes according to histological parameters of cellular atypia, stromal cellularity, stromal pattern, mitotic index and nature of the margins (2). About 10–15% of PTs are malignant, of which only 10–26% develop metastases (8). Clinically, most PT presents as a palpable firm, smooth, painless, mobile mass that usually grows rapidly within just a few weeks. In general, it is difficult to distinguish between PTs and FA clinically. PTs should be suspected in any rapidly growing breast mass, even in younger patients. On physical examination, PTs are usually hard, well-defined, mobile, does not adhere to skin masses, and have a median size of about 4 cm. Unlike FA, PTs have a tendency to recur and progress even though they are histologically benign. Molecular evidence has been used to describe the progression from FA to PTs. It is difficult to distinguish PTs and FA on a macro level, and even more difficult to distinguish their subtypes. The definitive diagnosis can only be made on histopathology after complete resection of the tumor.
Strengths and limitations
Mammography may reveal a dense, smooth, nonspiculated, and polylobulated masses that are nonspecific and difficult to differentiate PTs from other tumors. Although only 20% of them give abnormal findings on screening mammography. On US, they appear as round/oval or irregular lesions with clear or unclear margins, homogeneous or heterogenous hypoechoic patterns, and with or without posterior enhanced echo. In addition, larger size, irregular shape, and the presence of cystic spaces are more correlate with malignant than benign PTs. Although MRI is extremely sensitive to the diagnosis of breast cancer, it is difficult to distinguish PTs from other breast tumors. On MRI, PTs are oval, well circumscribed and isointense on T1-weighted images and heterogeneous hyperintense on T2-weighted images. MRI showing heterogeneous internal structure and non-enhanced separation may be more suggestive of the diagnosis of PTs (9).
PTs is a challenge for pathologists and surgeons. It is unreliable to distinguish PTs from FA by fine needle aspiration cytology (FNAC). CNB is a highly sensitive method for preoperative diagnosis of breast cancer, and is widely used in clinic (10). However, the small sample size obtained for CNB renders it difficult to make a definitive diagnosis of PTs.
On gross examination, PTs mimic the FA, is a well-defined lobulated mass with raised edges. However, small sacs or narrow fissures are visible on the section, ranging in color from grayish yellow to tan. Histologically, PTs are tumors of interstitial hypergrowth of fibrous and epithelial biphasic differentiation. PTs are classified as benign, borderline, and malignant according to histological features, including infiltrative margins, stromal overgrowth, mitotic counts, cell proliferation, and atypia. Benign PTs show minimal cell atypia, slightly increased stromal cells and mitotic index ≤4/10 HPF, which distinguished them from FA. Borderline PTs are characterized by microscopic tumor invasion, focal stromal hyperplasia, moderate stromal cells, moderate stromal cell atypia, and no malignant heterologous components. Mitotic activity is in the range of 5–9/10 HPF. Malignant PTs show marginal infiltration, stromal overgrowth, significant atypia of stromal cells, and greater than 10/10 HPF mitosis (11).
However, our patient had multiple PTs. Bilateral breast mass resection (outer lower quadrant of left breast, measured 5.4 cm × 4.4 cm; upper outer quadrant of right breast, measured 2.2 cm × 1.5 cm) was performed in 2014. Postoperative pathology showed benign PTs of both breasts without margins status. Among them, the small tumor in the right breast did not recur after resection. In June 2018, 4 years and 4 months after surgery, a dove egg-sized tumor was found on the outer lower quadrant of the left breast, which increased to 10.0 cm × 8.0 cm in half a year. The tumor was considered to be a local recurrence of left breast PT, and left breast lumpectomy was performed. Intraoperative frozen pathology showed a left breast fibroepithelial tumor, and the postoperative pathology was benign PT. Six months after that, an egg-sized mass was found on the outside of the left breast, which gradually increased to 15 cm in diameter. The physical examination revealed a significant enlargement of the left breast due to a large mass occupying majority of the breast. The mass was firm, nontender and mobile from the chest wall. The covering skin was attenuated with the apparent dilation of subcutaneous veins. The pathology of CNB specimens showed fibroepithelial lesions with cell proliferation. It is especially difficult to diagnose and determine the classification of breast PTs when it is detected by CNB. The tumor was diagnosed as being a borderline PT by postoperative pathology. The surgical margin was not involved by the tumor. After 24 months of follow-up, there was no evidence of local or distant recurrence. This report also explains extensive resection of giant PTs (greater than 10 cm in diameter) can help prevent local recurrence, and small benign PTs (less than 3 cm in diameter) without marginal status can be performed with a “watchful waiting” strategy.
Comparison with similar researches
As the rare tumor of the breast, PTs pose a great challenge for surgeons. Local recurrence of PTs is more common than distant metastasis. The initial evaluation of PTs relies on a triple evaluation of radiological, clinical, and histological examination. Surgical excision is the principal treatment for PTs. PTs are difficult to distinguish from other breast tumors before surgery. Marginal negative surgery remains the primary treatment for PTs. Study has shown that there is no statistically significant difference in recurrence rates between mastectomy and lumpectomy patients (12). The goal is always to strike a balance between preserving cosmetic results and function with the risk of recurrence. However, local recurrence of PTs is more common than distant metastases. It has been reported that the local recurrence rate of benign PTs is approximately 8% and that of borderline PTs is 21%, and the risk of malignant tumor transformation is increased by about 8% per recurrence (13). Histologically, recurrent tumors are basically the same as primary tumors, or have a malignant tendency. Surgery with negative margins is the primary treatment for PTs. Based on retrospective data, current guidelines recommend extensive local excision (≥1 cm margin) for malignant or borderline PTs and excisional biopsy for benign PTs, regardless of tumor size. There is a lack of definitive guidelines for the resection and postoperative surveillance of PTs.
Explanations of findings
In current practice, not all patients are treated according to current guidelines. The adequacy of the incisal margin is also controversial. A study of Yom et al. concluded that the treatment effect of 0.1 mm clear margin and 1 cm clear margin is equivalent (14). Onkendi et al. has shown that the extent of surgical resection in patients with borderline and malignant PTs did not affect patients’ disease-free survival (15). Benign and borderline PTs are less aggressive and have a lower recurrence rate regardless of the status of the resection margin (16). Positive margins are not associated with distant metastases and are only associated with an increased risk of local recurrences. A recent study has shown the lack of correlation between disease recurrence and margin width (17).
Implications and actions needed
Clear margins are recommended for benign PTs, but re-excision is not mandatory if the margins are involved. Benign PTs are usually indistinguishable from FA based on CNB and can only be diagnosed after resection, usually without attention to the status margins. Once the diagnosis has been made, the dilemma of whether to operate again to obtain a negative margin or wait-and-watch is often faced by breast surgeons. An entire cohort study by Rosenberger et al. found that wider margins were not associated with a reduced risk of local recurrence. Regardless of the margin width, they do not recommend re-excision for benign PTs to obtain a negative margin, because an enlarged surgical margin is unlikely to reduce local recurrence (18). Borderline PTs should be negative margins because of the risk of recurrence and the possibility of evolve to malignant PTs after recurrence (3). In malignant PTs, 3 mm margin could not reduce recurrence. There is weak evidence that a margin of 1 mm may be sufficient (19). Although most surgeons are uncomfortable with PTs with a positive margin, it is reasonable for benign PTs to adopt “watch and wait” strategy. Recent findings suggest that close radiological and clinical follow-up may provide a better management process than re-excision when the margins of benign and borderline PTs are positive (20).
Conclusions
The current recommendation that PTs should be extensively resected regardless of tumor size might be revised.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-84/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-84/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-84/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee (s) and with the Helsinki Declaration (as revised in 2013). Ethical approval was granted by the hospital’s ethics committee (No. YN2023-038-01). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhou ZR, Wang CC, Yang ZZ, et al. Phyllodes tumors of the breast: diagnosis, treatment and prognostic factors related to recurrence. J Thorac Dis 2016;8:3361-8. [Crossref] [PubMed]
- Lakhani SR, Ellis IO, Schnitt SJ, et al. World Health Organization (WHO) Classification of tumours of the breast. WHO Classification of Tumours, 4th edition, vol. 4, International Agency for Research on Cancer (IARC) Press, Lyon, France, 2012, 143-7.
- Spitaleri G, Toesca A, Botteri E, et al. Breast phyllodes tumor: a review of literature and a single center retrospective series analysis. Crit Rev Oncol Hematol 2013;88:427-36. [Crossref] [PubMed]
- Mituś JW, Blecharz P, Walasek T, et al. Treatment of Patients with Distant Metastases from Phyllodes Tumor of the Breast. World J Surg 2016;40:323-8. [Crossref] [PubMed]
- Lu Y, Chen Y, Zhu L, et al. Local Recurrence of Benign, Borderline, and Malignant Phyllodes Tumors of the Breast: A Systematic Review and Meta-analysis. Ann Surg Oncol 2019;26:1263-75. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Breast cancer (version 2. 2016). Available online: https://www.nccn.org/professionals/physician_ gls/pdf/breast.pdf.
- Sawalhi S, Al-Shatti M. Phyllodes tumor of the breast: a retrospective study of the impact of histopathological factors in local recurrence and distant metastasis. Ann Saudi Med 2013;33:162-8. [Crossref] [PubMed]
- Koh VCY, Thike AA, Nasir NDM, et al. Size and heterologous elements predict metastases in malignant phyllodes tumours of the breast. Virchows Arch 2018;472:615-21. [Crossref] [PubMed]
- Wurdinger S, Herzog AB, Fischer DR, et al. Differentiation of phyllodes breast tumors from fibroadenomas on MRI. AJR Am J Roentgenol 2005;185:1317-21. [Crossref] [PubMed]
- Cheng SP, Chang YC, Liu TP, et al. Phyllodes tumor of the breast: the challenge persists. World J Surg 2006;30:1414-21. [Crossref] [PubMed]
- Tan BY, Acs G, Apple SK, et al. Phyllodes tumours of the breast: a consensus review. Histopathology 2016;68:5-21. [Crossref] [PubMed]
- Tan PH, Thike AA, Tan WJ, et al. Predicting clinical behaviour of breast phyllodes tumours: a nomogram based on histological criteria and surgical margins. J Clin Pathol 2012;65:69-76. [Crossref] [PubMed]
- Yilmaz E, Sal S, Lebe B. Differentiation of phyllodes tumors versus fibroadenomas. Acta Radiol 2002;43:34-9. [PubMed]
- Yom CK, Han W, Kim SW, et al. Reappraisal of conventional risk stratification for local recurrence based on clinical outcomes in 285 resected phyllodes tumors of the breast. Ann Surg Oncol 2015;22:2912-8. [Crossref] [PubMed]
- Onkendi EO, Jimenez RE, Spears GM, et al. Surgical treatment of borderline and malignant phyllodes tumors: the effect of the extent of resection and tumor characteristics on patient outcome. Ann Surg Oncol 2014;21:3304-9. [Crossref] [PubMed]
- Sevinç Aİ, Aksoy SÖ, Güray Durak M, et al. Is the extent of surgical resection important in patient outcome in benign and borderline phyllodes tumors of the breast? Turk J Med Sci 2018;48:28-33. [Crossref] [PubMed]
- Jang JH, Choi MY, Lee SK, et al. Clinicopathologic risk factors for the local recurrence of phyllodes tumors of the breast. Ann Surg Oncol 2012;19:2612-7. [Crossref] [PubMed]
- Rosenberger LH, Thomas SM, Nimbkar SN, et al. Contemporary Multi-Institutional Cohort of 550 Cases of Phyllodes Tumors (2007-2017) Demonstrates a Need for More Individualized Margin Guidelines. J Clin Oncol 2021;39:178-89. [Crossref] [PubMed]
- Ofri A, Stuart KE, Chan B, et al. Diagnosis and management of phyllodes tumours for the surgeon: An algorithm. Surgeon 2022;20:e355-65. [Crossref] [PubMed]
- Genco IS, Purohit V, Hackman K, et al. Benign and borderline phyllodes tumors of the breast: Clinicopathologic analysis of 205 cases with emphasis on the surgical margin status and local recurrence rate. Ann Diagn Pathol 2021;51:151708. [Crossref] [PubMed]
Cite this article as: Zhang X, Gan L, Zhao J, Zhang H. Recurrent giant phyllodes tumor of the breast: a case report. AME Case Rep 2024;8:39.