
Endobronchial ultrasound-guided cautery-assisted transbronchial mediastinal cryobiopsy in the diagnosis of fibrosing mediastinitis secondary to atypical sarcoidosis: a case report
Highlight box
Key findings
• In this report, we reported a fibrosing mediastinitis (FM) secondary to atypical sarcoidosis case. The clinical symptoms are not specific, and diagnosis and treatment should be made as soon as possible to reduce the occurrence of complications.
What is known and what is new?
• FM secondary to atypical sarcoidosis is rarely reported at home and abroad, which require pathological confirmation before diagnosis.
• In this report, we report a patient who was admitted to the hospital with FM secondary to atypical sarcoidosis diagnosed by endobronchial ultrasound-guided cautery-assisted transbronchial mediastinal cryobiopsy (EBUS-CA-TBMCB), which is giant rarely.
What is the implication, and what should change now?
• FM is a rare syndrome characterized by excessive hyperplasia of fibrous tissue in the mediastinum.as an improved minimally invasive method, EBUS-CA-TBMCB cannot only avoid more invasive surgery but also obtain enough tissue samples, which can be used as the effective biopsy method for the etiology of FM.
Introduction
Fibrosing mediastinitis (FM) is considered to be a clinicopathological syndrome rather than a single disease. It is a mediastinal hyperfibrosis reaction caused by many causes (1). The lesion mainly involved the middle mediastinum and the periphery of bilateral pulmonary hili. With the progress of the disease, the lesion may compress or block adjacent tissues and organs such as bronchi, superior vena cava, pulmonary vessels, and esophagus, which may cause corresponding clinical symptoms (2). However, the etiology of FM varies from region to region in the world (3). In high-income countries (like North America), the primary causes of FM are histoplasmosis and sarcoidosis (4,5). On the contrary, in developing countries, such as China, tuberculosis is a common cause of FM (6). FM secondary to atypical sarcoidosis is rarely reported at home and abroad. Its clinical manifestations represent a lack of specificity, and the initial diagnosis is frequently difficult. We present this article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-160/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 70-year-old man was hospitalized with a cough and dyspnea for two months on December 27, 2020. In October 2020, the patient accepted no obvious inducement to cough, accompanied by dyspnea. Chest computed tomography (CT) in a hospital in Chongqing showed multiple solid nodules and consolidation in both lungs, some of which were located under the pleura. Interstitial changes in both lungs might be scattered with inflammation. Enlarged lymph nodes and partial calcification were seen in the hilar and mediastinum of both lungs. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) was performed in the group seven lymph nodes. A rapid on-site evaluation (ROSE) smear showed some epithelioid cells and lymphocytes. Pathology showed that punctured lymph tissue was locally squeezed, and no tumor cells were found. The pulmonary function indicates moderate obstructive pulmonary ventilation dysfunction and pulmonary diffusion dysfunction. The symptoms did not improve after treatment with ceftizoxime (2 g intravenous infusion every 12 h) for anti-infection and bronchodilation. The patient has been smoking for about 30 years, with an average of 20 cigarettes/per day. He has quit smoking for more than 5 years and does not drink. Simultaneously, there was no pulmonary tuberculosis, no history of coronary heart disease, hypertension, and diabetes, no history of dust and radiation exposure, and no family genetic disease.
The initial physical examination showed a heart rate of 95 beats/min, a respiratory rate of 26 breaths/min, a body temperature of 36.1 ℃, and a blood pressure of 14.13/9.87 kPa. The body mass index (BMI) was 21 kg/m2. Oxygenation index (PaO2/FiO2) 206 mmHg, the lips are slightly cyanotic, the jugular vein is undistended, the thorax is normal, bilateral respiratory movements are symmetrical and bilateral speech fibrillation is weakened. Percussion of both lower lungs showed dullness, low-pitched moist rales could be heard in both lungs. The heart rhythm was uniform, the heart sounds were not significantly enhanced or weakened, and no murmur was heard in each valve area. The abdomen is soft, the liver and spleen are not under the ribs, the lower limbs are slightly edematous, and no clubbing fingers are found.
The laboratory testing revealed the following: white blood cells (WBC) count, 7.62×109/L; neutrophil percentage, 78.3%; procalcitonin, 1.03 ng/mL; C-reactive protein (CRP), 94.8 mg/L; interleukin-6 (IL-6), 19.2 pg/mL; serum albumin, 32.5 g/L; D-dimer, 2.99 mg/L; arterial blood gas (FiO2, 33%; pH, 7.46; PaCO2, 35 mmHg; PaO2, 68 mmHg; HCO3−, 24.9 mmol/L; lactic acid, 1.1 mmol/L; brain natriuretic peptide (BNP) 1,640.00 pg/mL. The following measures and indicators were normal: Liver and kidney function, myocardial enzyme spectrum, stool routine, tuberculosis antibody screening, blood IgG, IgA, IgM, IgE, IgG4, lymphocyte subsets, acquired immune deficiency syndrome (AIDS), hepatitis C and syphilis antibodies, hepatitis B surface antigen, hepatitis B e antigen, tuberculosis infection T cell spot test (T-SPOT), anti-cyclic citrulline peptide antibody, complement C3, complement C4, rheumatoid factor, antineutrophil cytoplasmic antibody (ANCA), anti-extractable nuclear antigen (ENA) antibody, anti myeloperoxidase (MPO) antibody, protease 3 (PR3) and glomerular basement membrane (GBM) antibody, anti ds-deoxyribonucleic acid (DNA) antibody, anticardiolipin antibody, anti-nuclear antibody, plasma 1-3-β-D glucan measurement, galactomannan antigen test, carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), alpha-fetoprotein (AFP), carbohydrate antigen (CA) 19-9, CA125, CA24-2, cytokeratin 19 (CYFRA211), and squamous cell carcinoma antigen (SCCA). In addition, the sputum acid-fast staining, sputum smear examination, and sputum bacterial and fungal culture were negative. Color Doppler ultrasound reveal the size of each chamber of the heart was normal, with mild pulmonary hypertension and mild pulmonary regurgitation.
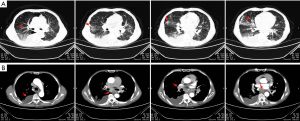
On the day of admission, the contrast-enhanced chest CT showed bronchial vascular bundles of both lungs are thickened, and both lungs have multiple solid nodules and a slight piece like consolidation of different sizes, distributed along the bronchial vascular bundles. Some of them are located under the visceral pleura, similar to pneumoconiosis nodules, but mainly in the middle and lower lungs (Figure 1A); multiple mediastinal enlarged lymph nodes with calcification lead to compression of the right pulmonary portal vessels and left atrium; bilateral parietal pleural thickening with bilateral pleural effusion, mainly on the right side (Figure 1B).
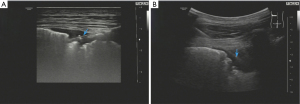
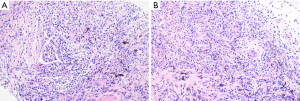
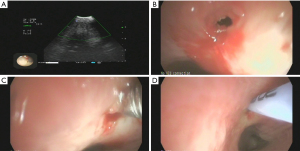
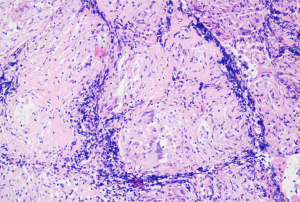
After admission, the patient was given 3L/min oxygen inhalation and administered piperacillin/tazobactam (4.5 g intravenous infusion every 8 h) for anti-infection, bronchodilator, and diuretic therapy, and bilateral thoracic puncture was performed for fluid extraction. The results of routine, biochemical, CEA, bacterial culture, and Xpert MTB/RIF in bilateral pleural effusion are shown in Table 1. After the above treatment, the patient’s cough and dyspnea were not significantly improved, and the chest CT was not significantly changed. Pulmonary infection after pulmonary edema secondary to heart failure cannot be explained. After obtaining the consent of the patient and his family members, the left parietal pleura and bilateral lower lung lesions were biopsied under ultrasound guidance (Figure 2A,2B). The pathological results of the left lower lung and left pleura showed fibrous tissue hyperplasia with more chronic inflammatory cell infiltration, and no typical granuloma lesions and malignant tumor tissue were found (Figure 3A); immunohistochemistry: CD34 (vascular+), CD68 (+), CD138 (−), CD38 (−), CD4 (−), CEA (−), CK-P (+), P40 (−), P53 (−), S-100 (−), SMA (+/−), TTF1 (+/−), IgG (−), IgG4 (−), special staining: acid-fast staining (AFS) (−), Giemsa staining (GMS) (−), periodic acid-Schiff staining (PAS) (−). The pathological biopsy of the right lung tissue presented chronic inflammation with interstitial fibrosis, and there were also no aspergillus hyphae, typical granulomatous lesions, and malignant tumor tissue that were found (Figure 3B); tubercle bacillus DNA (TB-DNA) were both negative. At this moment, we preliminarily ruled out pulmonary tuberculosis, aspergillosis, cryptococcosis, systemic lupus erythematosus, rheumatoid arthritis, pulmonary vasculitis, and IgG4 syndrome. At this time, we considered that it might be related to the FM that affected the pulmonary venous return, and its cause was still highly suspected of sarcoidosis. Therefore, we performed the biopsy again on the mediastinal lesions. EBUS-guided cautery-assisted transbronchial mediastinal forceps biopsies (EBUS-Ca-TBMFB) was performed as follows: we placed EBUS-convex probe (Olympus BF260, from Japan) outside the diseased bronchus, and used ultrasound to detect the scope of the lesion. After avoiding larger blood vessels, we used a needle electrosurgery (Erbo, from Germany) to cut it layer by layer. Branch the tracheal wall, with a size of about 2 mm, and introduce the biopsy forceps through the ultrasound bronchoscope biopsy hole (Figure 4A-4C). However, the amount of forceps was insufficient during the operation, So we replaced it with a liquid nitrogen cryoprobe with an outer diameter of 1.1mm, and entered the mediastinum target area at the incised bronchial wall, and monitored the whole process with ultrasound. After the cryoprobe contacts the target tissue, it rapidly freezes the surrounding tissue by releasing liquid nitrogen. After freezing for 3–5 seconds, remove the cryoprobe and ultrasonic bronchoscope from the airway together, put the frozen specimen into formalin solution, and conduct ultrasonic bronchoscopy again to check that there is no obvious bleeding in the airway and mediastinum. The cryobiopsy is then repeated again until the specimen is satisfactory and the operation is stopped (Figure 4D). Postoperative pathology showed non caseous granulomatous lesion with no fungal filaments or spores, and fungal immunofluorescence staining was negative, which was consistent with sarcoidosis (Figure 5); special staining: reticular fiber (+), AFS (−), GMS (−), PAS (−); TB-DNA was negative. So far, the FM diagnosis secondary to sarcoidosis is clear, so we provided the patient prednisone orally at the initial dose of 0.4 mg/kg/d, and continued to give oral torasemide diuretic treatment. After one week, the patient’s cough and dyspnea improved significantly, and the oxygen permeability was 95% without oxygen inhalation, and then discharged from the hospital.
Table 1
| Variable | Left | Right | Reference range |
|---|---|---|---|
| Colour | Yellow | Yellow | / |
| Transparency | Turbid | Turbid | / |
| Rivalta test | − | − | / |
| Total number of cells (×106/L) | 4,256 | 4,100 | / |
| WBC (×106/L) | 3,640 | 2,400 | / |
| Multinucleate cell (%) | 65 | 55 | / |
| Monocyte (%) | 35 | 45 | / |
| Total protein (g/L) | 54.9 | 55.6 | 65.0–85.0 |
| Albumin (g/L) | 18.1 | 19.4 | 40.0–55.0 |
| Globulin (g/L) | 36.8 | 36.2 | 20.0–40.0 |
| Albumin/globulin | 0.49 | 0.54 | 1.20–2.40 |
| Adenosine deaminase (U/L) | 20 | 19 | 4.0–18.0 |
| Glucose (mmol/L) | 6.43 | 6.52 | 3.9–6.0 |
| Lactate dehydrogenase (U/L) | 319 | 248 | 100.0–245.0 |
| Transferrin (g/L) | 0.8 | 0.6 | 2.0–4.0 |
| CEA (ng/mL) | 1.4 | 0.53 | 0.00–5.00 |
| Bacterial culture | − | − | − |
| Xpert MTB/RIF | − | N | − |
WBC, white blood cells; CEA, carcinoembryonic antigen; “−” means negative; “N” means no test.
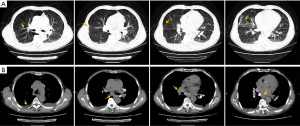
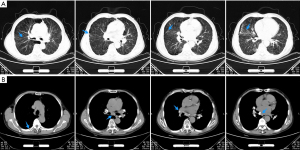
After discharge, we continue to follow up with the patient, and his cough and dyspnea were further improved,and the finger pulse oxygen permeability was over 98% without oxygen inhalation. On April 2, 2021, the review of chest CT showed that the thickened bronchovascular bundle decreases, and bilateral pulmonary nodules and consolidation become significantly smaller or less (Figure 6A). At the same instant, mediastinal lymph nodes were significantly reduced, the compression of right hilar blood vessels and left atrium was improved, and the right pleural effusion was significantly reduced (Figure 6B). Next, we suggest that prednisone be gradually reduced to complete withdrawal, with a total course of treatment of 6 months. On October 18, 2021, he returned to the hospital again to recheck chest CT, and the results showed that the bronchial vascular bundle is clear, and pulmonary consolidation and most nodules disappear completely (Figure 7A). What is more worth celebrating is that the enlarged mediastinal lymph node was further reduced, no obvious pressure was found on the right pulmonary hilum and left atrium, and pleural effusion completely disappeared (Figure 7B).
Discussion
Up to now, the pathophysiological mechanism of FM is not clear, and it has the characteristics of concealed attack and progressive course (7). FM can be divided into the localized type (localized soft tissue mass, often accompanied by calcification) and diffuse type (diffuse infiltrative mass, no capsule, unclear boundary, generally no cystic change or necrosis) according to the typical manifestations of CT, and the pathological types are granulomatous type and non-granulomatous type (8). Granulomatous FM is mostly related to tuberculosis, fungi, histoplasmosis infection, or sarcoidosis, while non-granulomatous FM is mostly caused by non-infectious factors, such as autoimmune diseases, drugs, dust, radiation, etc., or it is difficult to identify the cause of the idiopathic disease (9). This case considered localized FM according to the signs of the contrast-enhanced chest CT. As mentioned above, the causes of FM vary from region to region in the world. In North America, the main causes of FM are histoplasmosis and sarcoidosis (4,5), while in China, many cases are related to tuberculosis (6). In this case, no evidence of Mycobacterium tuberculosis infection was obtained in tuberculosis infection T-SPOT, sputum acid-fast staining, left pleural fluid Xpert MTB/RIF, pleural fluid biochemistry, bilateral lung, and pleural pathological biopsies, and glucocorticoids were used for half a year without intrapulmonary dissemination of tuberculosis. Therefore, tuberculosis was not considered. Interestingly, no typical sarcoidosis lesion was found in the pathology of the left pleural and lung puncture biopsy, but it was confirmed as sarcoidosis by endobronchial ultrasound-guided cautery-assisted transbronchial mediastinal cryobiopsy (EBUS-CA-TBMCB). We think there are the following reasons. First of all, this case has multiple pulmonary nodules with mediastinal lymph node enlargement and bilateral pleural effusion, and pulmonary fibrosis still exists after treatment, which is inconsistent with any clinical stage of pulmonary sarcoidosis (10), and belongs to atypical sarcoidosis, thus interfering with our judgment. Secondarily, sarcoidosis is a multisystem granulomatous disease of unknown etiology. Although the lung is the most common organ of sarcoidosis, with a frequency of about 90%, it rarely involves the pleura (11,12), so the positive rate of the pleural biopsy for diagnosis of sarcoidosis is low.
The clinical manifestations of patients with FM principally depend on the influence of the mediastinal structure, such as when the esophagus is compressed, the patient may have difficulty swallowing. If the bronchus is compressed, it may cause obstructive ventilation function (13). Obstruction or compression of pulmonary vessels in the mediastinum ordinarily involves the superior vena cava, pulmonary vein, and pulmonary artery, while the pericardium, coronary artery, aorta, and aortic branch vessels are rarely involved (14,15). Patients with pulmonary vein obstruction may have progressive or exertional dyspnea, cough, and hemoptysis, which are called “pseudo mitral stenosis syndrome” (16). Long-term pulmonary vein obstruction can also lead to secondary pulmonary hypertension and pulmonary heart disease, accompanied by pulmonary interstitial edema and pleural effusion (17). The patient’s clinical manifestations endure mainly cough and dyspnea, accompanied by bilateral pleural effusion, pulmonary function showed moderate obstructive pulmonary ventilation dysfunction, and pulmonary diffusion dysfunction and cardiac ultrasound showed pulmonary hypertension, which was consistent with the above studies. It is worth mentioning that this patient sustains bilateral pleural effusion. We consider the cause is not only the increase of pulmonary capillary hydrostatic pressure and chronic pulmonary hypertension after pulmonary vein compression (18-20) but also the lymphatic obstruction of sarcoidosis granulomatous and sarcoidosis granulomatous inflammation directly involving the pleura (21). Sarcoidosis-associated pleural effusion occurs in about 1% of sarcoidosis patients. The disease most often occurs in the initial manifestation of sarcoidosis or within the first year. It is a usually turbid exudate, and dyspnea is the most common symptom (22), which is also consistent with the disease course, clinical symptoms and exudative pleural effusion (23) of our case.
The diagnosis of FM is based on typical imaging findings. The contrast-enhanced chest CT. is the most preferred method for diagnosis and evaluation of suspected or known cases of FM (8). On the contrast-enhanced chest CT, FM usually shows localized or diffuse soft tissue density shadows in the mediastinum and around the pulmonary hilum, with an irregular shape, with or without calcification, and the most common is to affect the middle mediastinum, while the front and rear mediastinum is rarely affected (8,13). If chest CT shows indirect signs such as exudation, thickened interlobular septum, and thickened bronchovascular bundle, it usually indicates pulmonary interstitial edema after pulmonary vein stenosis (8,24). The imaging findings of this case are consistent with the above literature reports. It should be noted that fluorine-18 fluorodeoxyglucose-positron emission tomography/CT (18F-FDG-PET/CT) cannot differentiate FM or malignant diseases, because both are related to high metabolic activity (8). Due to the interference of clinical symptoms and pulmonary function, this case was misdiagnosed as chronic obstructive pulmonary disease outside the hospital. The contrast-enhanced chest CT was performed after admission, and radiologists and clinicians failed to recognize the disease in time at an initial stage, indicating that we were not alert to the disease. Fortunately, the diagnosis was finally confirmed through multiple pathological biopsies of multiple sites.
The pathological biopsy is extremely important for the etiological diagnosis of mediastinal lesions. EBUS-TBNA is a minimally invasive method to replace mediastinoscopy in the diagnosis of mediastinal lesions, with high sensitivity and specificity (25,26). However, the cytological pathology obtained may limit the diagnostic efficacy of granulomatous diseases (25,27,28). Therefore, EBUS-CA-TBMCB has become a new research hotspot for the pathological sampling of mediastinal lesions. Zhang et al. (29) conducted a randomized trial, which included a total of 197 patients receiving EBUS-TBNA and EBUS-CA-TBMCB. In their experimental results, the total diagnostic rates of EBUS-TBNA and EBUS-CA-TBMCB were 79.9% and 91.8%, respectively. In benign diseases, EBUS-CA-TBMCB was more sensitive than EBUS-TBNA. In this case, the cause was not clear through EBUS-TBNA outside the hospital, so in the preceding stage of diagnosis after admission to our hospital, EBUS-Ca-TBMFB was performed firstly, but the target site was extremely hard, and the forceps could not obtain enough samples for histopathology, and then the samples were taken successfully after the use of cryobiopsy.
The treatment of FM is various due to various primary etiologies, and infectious diseases still advocate chemical drug treatment for pathogens. However, the treatment of non-infectious diseases needs to be evaluated in stages. It can be observed clinically in the early stage. In the later stage, if there is compression of adjacent organs, such as pulmonary vessels or bronchi, surgery or vascular intervention is required (2,30,31). Glucocorticoid treatment is limited to FM secondary to sarcoidosis (32,33). The patient received oral prednisone treatment for half a year, and the chest CT showed that the pulmonary nodules and consolidation were completely absorbed, bilateral pleural effusion disappeared, and the mediastinal and bilateral hilar lymph nodes were significantly reduced, which also confirmed the diagnosis of sarcoidosis.
Conclusions
To sum up, FM is a rare syndrome characterized by excessive hyperplasia of fibrous tissue in the mediastinum. Clinicians have insufficient awareness and vigilance of FM, which is easy to cause misdiagnosis and missed diagnosis. The diagnosis of FM is mainly based on typical imaging manifestations, that is, the density shadows of the soft tissues around the mediastinum and pulmonary hilum, which are irregular in shape, with or without calcification. Although EBUS-TBNA has proved to be minimally invasive and effective in the diagnosis of mediastinal lesions, there is a shortage of tissue samples in the diagnosis of granulomatous diseases. Therefore, as an improved minimally invasive method, EBUS-CA-TBMCB can not only avoid more invasive surgery but also obtain enough tissue samples. In addition, the existing literature reports that its complications are similar to EBUS-TBNA, which can be used as the effective biopsy method for the etiology of FM. However, its effectiveness and safety still need to be verified by multi-center and prospective studies in the future.
Acknowledgments
We would like to thank Dr. Lingxu Wang for his technical guidance in the picture modification of the manuscript.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-160/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-160/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-160/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Peikert T, Colby TV, Midthun DE, et al. Fibrosing mediastinitis: clinical presentation, therapeutic outcomes, and adaptive immune response. Medicine (Baltimore) 2011;90:412-23. [Crossref] [PubMed]
- Jain N, Chauhan U, Puri SK, et al. Fibrosing mediastinitis: when to suspect and how to evaluate? BJR Case Rep 2016;2:20150274. [Crossref] [PubMed]
- Kobayashi Y, Ishiguro T, Takaku Y, et al. Clinical Features of Fibrosing Mediastinitis in Japanese Patients: Two Case Reports and a Literature Review. Intern Med 2021;60:3765-72. [Crossref] [PubMed]
- Sherrick AD, Brown LR, Harms GF, et al. The radiographic findings of fibrosing mediastinitis. Chest 1994;106:484-9. [Crossref] [PubMed]
- Loyd JE, Tillman BF, Atkinson JB, et al. Mediastinal fibrosis complicating histoplasmosis. Medicine (Baltimore) 1988;67:295-310. [Crossref] [PubMed]
- Hu Y, Qiu JX, Liao JP, et al. Clinical Manifestations of Fibrosing Mediastinitis in Chinese Patients. Chin Med J (Engl) 2016;129:2697-702. [Crossref] [PubMed]
- Eshak N, Abdelnaby M, Shehata H, et al. A case of fibrosing mediastinitis complicated by pulmonary hypertension and right-sided heart failure. Eur Heart J 2019;40:742. [Crossref] [PubMed]
- Garin A, Chassagnon G, Tual A, et al. CT features of fibrosing mediastinitis. Diagn Interv Imaging 2021;102:759-62. [Crossref] [PubMed]
- Xu Y, Xu W, Liu Y, et al. Pulmonary hypertension associated with combined fibrosing mediastinitis and bronchial anthracofibrosis: A retrospective analysis in a single Chinese hospital. Clin Respir J 2018;12:1134-40. [Crossref] [PubMed]
- Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2020;201:e26-51. [Crossref] [PubMed]
- Pierce TB, Margolis M, Razzuk MA. Sarcoidosis: still a mystery? Proc (Bayl Univ Med Cent) 2001;14:8-12. [Crossref] [PubMed]
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160:736-55. [PubMed]
- Garrana SH, Buckley JR, Rosado-de-Christenson ML, et al. Multimodality Imaging of Focal and Diffuse Fibrosing Mediastinitis. Radiographics 2019;39:651-67. [Crossref] [PubMed]
- McNeeley MF, Chung JH, Bhalla S, et al. Imaging of granulomatous fibrosing mediastinitis. AJR Am J Roentgenol 2012;199:319-27. [Crossref] [PubMed]
- Chazova I, Robbins I, Loyd J, et al. Venous and arterial changes in pulmonary veno-occlusive disease, mitral stenosis and fibrosing mediastinitis. Eur Respir J 2000;15:116-22. [Crossref] [PubMed]
- Harman M, Sayarlioglu M, Arslan H, et al. Fibrosing mediastinitis and thrombosis of superior vena cava associated with Behçet's disease. Eur J Radiol 2003;48:209-12. [Crossref] [PubMed]
- Bays S, Rajakaruna C, Sheffield E, et al. Fibrosing mediastinitis as a cause of superior vena cava syndrome. Eur J Cardiothorac Surg 2004;26:453-5. [Crossref] [PubMed]
- Beaudoin S, Gonzalez AV. Evaluation of the patient with pleural effusion. CMAJ 2018;190:E291-5. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015;46:903-75. [Crossref] [PubMed]
- Routsi C, Charitos C, Rontogianni D, et al. Unilateral pulmonary edema due to pulmonary venous obstruction from fibrosing mediastinitis. Int J Cardiol 2006;108:418-21. [Crossref] [PubMed]
- Huggins JT, Doelken P, Sahn SA, et al. Pleural effusions in a series of 181 outpatients with sarcoidosis. Chest 2006;129:1599-604. [Crossref] [PubMed]
- Chopra A, Foulke L, Judson MA. Sarcoidosis associated pleural effusion: Clinical aspects. Respir Med 2022;191:106723. [Crossref] [PubMed]
- Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972;77:507-13. [Crossref] [PubMed]
- Sinha D, Kundaragi NG, Kale SK, et al. Fibrosing mediastinitis mimicking as chronic pulmonary thromboembolism. BJR Case Rep 2020;6:20190049. [Crossref] [PubMed]
- Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and oesophageal endosonography for the diagnosis and staging of lung cancer. European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Eur Respir J 2015;46:40-60. [Crossref] [PubMed]
- Mondoni M, D'Adda A, Terraneo S, et al. Choose the best route: ultrasound-guided transbronchial and transesophageal needle aspiration with echobronchoscope in the diagnosis of mediastinal and pulmonary lesions. Minerva Med 2015;106:13-9. [PubMed]
- Wahidi MM, Herth F, Yasufuku K, et al. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest 2016;149:816-35. [Crossref] [PubMed]
- Labarca G, Sierra-Ruiz M, Kheir F, et al. Diagnostic Accuracy of Endobronchial Ultrasound Transbronchial Needle Aspiration in Lymphoma. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc 2019;16:1432-9. [Crossref] [PubMed]
- Zhang J, Guo JR, Huang ZS, et al. Transbronchial mediastinal cryobiopsy in the diagnosis of mediastinal lesions: a randomised trial. Eur Respir J 2021;58:2100055. [Crossref] [PubMed]
- Cimenoglu B, Ozkan B, Basaran M, et al. Pulmonary Arterial Bypass Surgery for Fibrosing Mediastinitis Causing Severe Pulmonary Hypertension. Ann Thorac Surg 2019;107:e411-3. [Crossref] [PubMed]
- Fender EA, Widmer RJ, Knavel Koepsel EM, et al. Catheter based treatments for fibrosing mediastinitis. Catheter Cardiovasc Interv 2019;94:878-85. [Crossref] [PubMed]
- Hasegawa K, Ohno S, Takada M, et al. Sarcoidosis complicated with major pulmonary artery obstruction and stenosis. Intern Med 2012;51:2775-80. [Crossref] [PubMed]
- Seferian A, Steriade A, Jaïs X, et al. Pulmonary Hypertension Complicating Fibrosing Mediastinitis. Medicine (Baltimore) 2015;94:e1800. [Crossref] [PubMed]
Cite this article as: Tang N, Tao T, Bao XL. Endobronchial ultrasound-guided cautery-assisted transbronchial mediastinal cryobiopsy in the diagnosis of fibrosing mediastinitis secondary to atypical sarcoidosis: a case report. AME Case Rep 2024;8:49.


