Granulomatous mastitis and pectoralis major muscle defect following polyacrylamide hydrogel injection: a case report and literature review
Highlight box
Key findings
• Polyacrylamide hydrogel (PAAG) can stimulate long-term complications including foreign-body granulomatous mastitis and pectoralis major muscle damage.
What is known and what is new?
• Adverse reactions about injected PAAG were commonly reported as breast pain and oedema, masses, asymmetry, gel migration, infection and inflammation.
• The article presented a rare case with foreign-body granulomatous mastitis and pectoralis major muscle damage, and subsequently confirmed complete symptoms resolution after characteristic surgical management in our center. Injected PAAG can diffuse in different layers and result in secondary inflammation, so the complete removal is extremely challenging.
What is the implication, and what should change now?
• PAAG injections can cause short-term and long-term adverse reactions, and are often difficult to treat.
• Surgical intervention is a preferred modality for the patients with chronic granulomatous mastitis after PAAG injection for breast augmentation.
Introduction
Polyacrylamide hydrogel (PAAG) gained popularity as a permanent soft tissue filler material for breast augmentation, following its introduction in the early 20th century for cosmetic applications (1). The most common reported PAAG adverse reactions were breast pain and oedema, palpable masses, asymmetry, gel migration, and infection (2-4). It is of note, concerns were raised regarding its safety as studies showed potential carcinogenicity and neurotoxicity (5). Therefore, the growing number of postoperative complications draw the attention to the possibility of general toxic manifestations and immune reactions caused by injected PAAG. Here, we reported a case who developed foreign-body granulomatous mastitis on her left breast and pectoralis major muscle defect following PAAG injection for breast augmentation and the PAAG removal surgery one year later, providing insights into therapeutic approaches for complications caused by injected PAAG. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-174/rc).
Case presentation
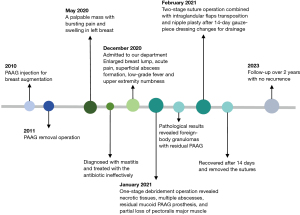
A 40-year-old female patient from Shanghai, China, was referred to Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine with an 8-month history of a painful and swollen mass in her left breast. In May 2020, she developed a palpable mass with bursting pain and swelling in her left breast, and was diagnosed with mastitis by a core needle biopsy and treated with antibiotic therapy ineffectively at another institution. When she was admitted to our department for further therapy in December 2020, she suffered from severe local symptoms, including enlarged breast lump, acute pain, and superficial abscess formation, and complained of the general symptoms, such as low-grade fever and upper extremity numbness. Previously, she had a cosmetic intervention of PAAG injection for breast augmentation in 2010 and subsequently underwent PAAG removal operation in 2011. The timeline of this case is depicted in Figure 1.
Upon the physical examination, the left breast appeared to be moderately larger than the right one, with the inverted nipple. A mass measuring 12 cm × 7.5 cm with hard texture and undefined boundaries was detected in the upper inner quadrant of the left breast, accompanied by overlying reddened skin measuring 4 cm × 3 cm. Enlarged lymph nodes were found in the left axilla.
Routine blood analysis revealed normal results with 6.46×109/L white blood cells, 57.00% neutrophils, and 3.36 mg/L C-reactive protein. Erythrocyte sedimentation rate was slightly elevated at 40.0 mm/h. Renal function, liver function and coagulation function demonstrated no obvious abnormalities. The microbiological culture of pus and PAAG was negative upon examination for pathogens.
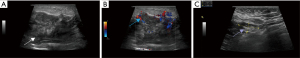
Ultrasonography revealed ill-defined heterogeneous echoes in the left breast. An anechoic or hypoechoic collection was seen posterior to the left areola and in the 8-4 o'clock clockwise direction of the breast, with a maximum depth of about 34 mm at about 11 o'clock involving the pectoralis major muscle (Figure 2A). Short linear blood flow signals around and inside the lesions were recorded by Color Doppler Flow Imaging (Figure 2B). Multiple enlarged and abnormal lymph nodes were detected in the left axilla, the largest one measuring 21 mm × 9 mm (Figure 2C).
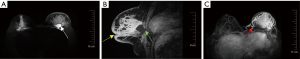
Contrast-enhanced magnetic resonance imaging (MRI) was routinely performed to assess range of the lesions. MRI depicted non-mass enhancement lesions in the multiregional distribution with vaguely defined boundaries in Breast Imaging-Reporting and Data System 4A (BI-RADS 4A), accompanied by edema in the retroglandular space and multiple enlarged lymph nodes in the ipsilateral axilla. T2-weighted sequence revealed residual PAAG as hyperintense collections (Figure 3A). Enhanced sagittal T1-weighted patterns were characterized as rapidly heterogeneous enhancement with affected nipple retraction, skin thickening, peripheral edema, and partial defect of the pectoralis major muscle (Figure 3B). Enlarged and circuitous blood vessels around the lesions also were noted (Figure 3C).
Through a periareolar radial incision, the patient underwent the surgical combination for treatment. Intraoperative observations of one-stage debridement operation revealed necrotic tissues, multiple abscesses, residual mucoid PAAG prosthesis diffused into the mammary glands and intramuscularly into the pectoralis major muscle, and partial loss of pectoralis major muscle. After the interval of two-week open gauze-piece dressing changes for drainage, we found that the lesions shrank with acute inflammation fully controlled, and then the patient underwent two-stage suture operation combined with intraglandular flaps transposition and nipple plasty to reduce recurrence rate and guarantee cosmetic satisfaction. Antibiotic clindamycin was administered postoperatively for 3 days, and traditional Chinese medicine was applied throughout the whole perioperative period. She recovered within 28 days to achieve the healing criteria with the absence of breast inflammatory manifestations and the disappearance of radiological findings from the one-stage operation, and no further complications were observed.
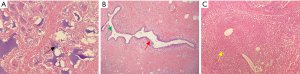
We performed an incisional biopsy for pathological confirmation. As shown in Figure 4, examination of the specimen revealed dilated mammary ducts infiltrated by gathered lymphocytes, plasma cells and neutrophils, and foreign-body granulomas caused by residual granular PAAG and microabscesses formation.
The patient was followed up by physical examination and ultrasound examination lasting for over 2 years, and recovered without further recurrence.
All procedures performed in this study were in accordance with the ethical standards of the institutional committee of Shuguang Hospital affiliated to Shanghai University of Traditional Chinese Medicine (approval No. 2019-702-57) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
PAAG is a nondegradable synthetic polymer, consisting of approximately 2.5% polyacrylamide and 97.5% water, and was once commonly applied for cosmetic soft-tissue augmentation as an ideal and safe biomaterial for long-term implantation (6). However, various adverse reactions associated with PAAG injection for augmentation mammaplasty were gradually reported, including lumps, mastalgia, asymmetry, infection and inflammation (3,7,8). Thus, the production and application of this material was banned in China from April 2006. Here, painful mass, gel migration, inflammation, continuous low-grade fever and upper extremity numbness were observed in our case. To the best of our knowledge, foreign-body granulomatous mastitis and pectoralis major muscle defect in a patient with a history of PAAG injection and removal was rarely reported.
PAAG injections can cause short-term and long-term adverse reactions. Studies have shown that long-term implantation of PAAG for augmentation mammaplasty elicited foreign-body chronic inflammation and granulomatous reactions (7,9,10). Foreign-body granuloma formation and inflammatory cell infiltration are the characteristic histopathological features after cosmetic augmentation mammaplasty with an injection of PAAG. Owing that breast inflammatory manifestations may obscure breast malignancies, distinguishing symptoms related to PAAG via imaging and histopathologic examinations should be prudent. Zhao et al. (11) presented a case of malignant breast tumor development following PAAG breast augmentation, demonstrating the possible correlation between PAAG injection and breast malignancy.
Certain factors, such as a tendency to gel migration, loose retromammary space, constant pectoralis major muscle contraction, and gravity, may allow PAAG to be carried through and rupture the connective breast tissues, chest wall, and abdominal wall along the retromammary space, causing local and general symptoms. Zhang et al. (12) reported a case with a 1-year history of a vulvar lump filled with subcutaneous fluid-filled regions 20 years after PAAG augmentation mammaplasty. As a foreign body, PAAG can form a fibrous capsule within a pseudocapsule, and damage the structure of the pectoralis major muscle and extrapleural space (13). Yang et al. (14) recently reported that PAAG gel diffused into the pectoralis major and minor muscles of 70.93% patients and intercostal muscles of 2.33% patients. To completely evaluate PAAG migration, He et al. (15) presented a retrospective review including patients with PAAG migration after injection from 2013 to 2018 and described a practical classification system ranging from type I to type V using MRI examinations for operative guidance.
As the literature and our case has shown, PAAG filler complications are often permanent and difficult to treat. The patients who underwent PAAG-injected breast augmentation, even had a PAAG removal operation afterwards, should have a routine breast examination physically and radiographically; however, once adverse complications occur, the appropriate therapeutic management must be determined for the individuals. Improper management of complications usually results in further secondary damage. Normally, patients with weak and non-specific symptoms are less motivated to seek medical care, but recurrent and persistent local and general symptoms may prompt patients to seek further treatment. Thus, establishing formal and standard therapeutic procedures for complications following PAAG injection is essential to shorten the healing time and avoid subsequent iatrogenic complications.
Considering the severity of the complications caused by PAAG injection, it is worth mentioning that adverse reactions should be carefully monitored and catalogued. In some cases with lumps at circumscribed one site, fine needle aspiration or an open procedure involving a small incision can be useful for removing the material (2,16). However, injected PAAG is likely to diffuse in layers and result in secondary inflammation, so the complete removal is extremely challenging (17). We found in our case that the hydrogel remained attached to the mammary glands, fascia, and pectoralis muscles, and even migrated through the extrapleural space, promoting a local and generalized inflammatory reaction. One of the primary long-term problems is characterized by the foreign-body granulomatous mastitis. The breast lesions appear as recurrent palpable masses, swelling, pain, abscesses and fistulas, possibly accompanied with general symptoms, including fever and upper extremity numbness. In such situations, Christensen et al. (18) recommended that granulomas should be treated with steroids and antibiotics or excision.
When conservative procedures fail, long-term granuloma-related complications were recommended to treated by surgical intervention (16). Woźniak-Roszkowska et al. (7) concluded that the patients diagnosed with autoimmune syndrome induced by adjuvants (ASIA), including local symptoms (breast lumps, pain, abscess and axilla pain) and general symptoms (recurrent low-grade fever, upper extremity numbness and sleep disturbances) after PAAG intra-breast injections have relieved symptoms severity after the surgical treatment. On the basis of the clinical manifestations of this patient, she presented symptoms consistent with major diagnostic criteria of ASIA, and subsequently confirmed complete symptoms resolution after surgical management in our center. When determining how to perform appropriate operation methods, Jin et al. (1) developed a surgical management protocol based on a practical classification guideline for patients with PAAG-injected breast augmentation. Under this protocol, the patient in our report presented type II complications and should accept open incision as a recommendation, which was consistent with our therapeutic protocol.
From our perspectives, we recommended all patients were routinely examined by ultrasound and enhanced MRI to assess the extent of the lesion preoperatively. For lumps with no local inflammatory manifestations, encapsulated gel material may be easily removed by fine needle aspiration or open surgery with small incisions; whereas chronic inflammation is present, surgical intervention is considered a preferred modality for management. It is extremely difficult to completely remove PAAG particles during the operation, but the residual prosthesis, necrotic breast tissue, abscess and fibrous capsule should be removed as much as possible to reduce further recurrence. We performed the characteristic stage operation for surgical management in this case, and two weeks of gauze pieces between one-stage debridement operation and two-stage suture operation were aimed to drain the pus and residual PAAG adequately, thereby to reduce the recurrence rate. Additionally, we suggested the PAAG material from aspiration, drainage and incision operation need to be routinely examined by microbiological culture to evaluate the presence of bacteria. Christensen et al. (18) revealed bacteria were identified in bacteriologically investigated samples (98%) from the patients with adverse reactions to polyacrylamide gel, even up to 5 years postinjection. For the patients with infectious nodules with a proven bacterial infection, appropriate antibiotics could take into consideration for treatment. However, more cases and clinical research are necessary to enrich the knowledge of therapeutic management strategies for PAAG injection-related complications.
Conclusions
PAAG for augmentation mammaplasty carries the risk of breast masses, migration, infection, inflammation, and damage to pectoralis muscles. Our case revealed that the granules remain adhered to the connective breast glands, fascia, and diffused intramuscularly into the pectoralis muscle, even after PAAG removal operation, further, PAAG can cause the long-term complications including foreign-body granulomatous mastitis and pectoralis major muscle defect. Effective assessment of local and general symptoms, routine follow-up examination and proper treatment therapies should be an obligatory aspect for patients with PAAG injection-related complications. Surgical intervention is a preferred modality for the patients with chronic inflammatory manifestations after PAAG injection for breast augmentation.
Acknowledgments
Funding: The article was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-174/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-174/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-174/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional committee of Shuguang Hospital affiliated to Shanghai University of Traditional Chinese Medicine (approval No. 2019-702-57) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jin R, Luo X, Wang X, et al. Complications and Treatment Strategy After Breast Augmentation by Polyacrylamide Hydrogel Injection: Summary of 10-Year Clinical Experience. Aesthetic Plast Surg 2018;42:402-9. [Crossref] [PubMed]
- Ono S, Ogawa R, Hyakusoku H. Complications after polyacrylamide hydrogel injection for soft-tissue augmentation. Plast Reconstr Surg 2010;126:1349-57. [Crossref] [PubMed]
- Qian B, Xiong L, Guo K, et al. Comprehensive management of breast augmentation with polyacrylamide hydrogel injection based on 15 years of experience: a report on 325 cases. Ann Transl Med 2020;8:475. [Crossref] [PubMed]
- Gierej P, Radziszewski M, Miłoński P, et al. Distal Hand Migration of Polyacrylamide Gel after Breast Augmentation: A Case Report and Review of the Literature. Indian J Plast Surg 2023;56:178-81. [Crossref] [PubMed]
- Patrick T. Polyacrylamide gel in cosmetic procedures: experience with Aquamid. Semin Cutan Med Surg 2004;23:233-5. [Crossref] [PubMed]
- Wang ZX, Luo DL, Dai X, et al. Polyacrylamide hydrogel injection for augmentation mammaplasty: loss of ability for breastfeeding. Ann Plast Surg 2012;69:123-8. [Crossref] [PubMed]
- Woźniak-Roszkowska E, Maślińska M, Gierej P, et al. Autoimmune syndrome induced by adjuvants after breast enhancement with polyacrylamide hydrogel: a study in Poland. Rheumatol Int 2020;40:1851-6. [Crossref] [PubMed]
- Leung KM, Yeoh GP, Chan KW. Breast pathology in complications associated with polyacrylamide hydrogel (PAAG) mammoplasty. Hong Kong Med J 2007;13:137-40. [PubMed]
- El-Shafey el-SI. Complications from repeated injection or puncture of old polyacrylamide gel implant sites: case reports. Aesthetic Plast Surg 2008;32:162-5. [Crossref] [PubMed]
- Park K, Nishiwaki F, Kabashima K, et al. A Case of Foreign-Body Granuloma of the Glabella due to Polyacrylamide Filler and an Intractable Ulcer after Skin Biopsy: An Immunohistochemical Evaluation of Inflammatory Changes. Case Rep Dermatol 2013;5:181-5. [Crossref] [PubMed]
- Zhao Y, Yuan NA, Li K, et al. Bilateral breast cancer following augmentation mammaplasty with polyacrylamide hydrogel injection: A case report. Oncol Lett 2015;9:2687-93. [Crossref] [PubMed]
- Zhang MX, Li SY, Xu LL, et al. Repeated lumps and infections: A case report on breast augmentation complications. World J Clin Cases 2019;7:3322-8. [Crossref] [PubMed]
- Margolis NE, Bassiri-Tehrani B, Chhor C, et al. Polyacrylamide gel breast augmentation: report of two cases and review of the literature. Clin Imaging 2015;39:339-43. [Crossref] [PubMed]
- Yang Y, Li S, He J, et al. Clinicopathological Analysis of 90 Cases of Polyacrylamide Hydrogel Injection for Breast Augmentation Including 2 Cases Followed by Breast Cancer. Breast Care (Basel) 2020;15:38-43. [Crossref] [PubMed]
- He J, Wang T, Dong J. Classification and Management of Polyacrylamide Gel Migration After Injection Augmentation Mammaplasty: A Preliminary Report. Aesthetic Plast Surg 2020;44:1516-21. [Crossref] [PubMed]
- Wei W. Treatment of complications from polyacrylamide hydrogel breast augmentation. Exp Ther Med 2016;12:173-6. [Crossref] [PubMed]
- Ebisudani S, Inagawa K, Suzuki Y, et al. Unilateral Breast Inflation Caused by Breastfeeding after Polyacrylamide Hydrogel Injection. Plast Reconstr Surg Glob Open 2021;9:e3335. [Crossref] [PubMed]
- Christensen L, Breiting V, Bjarnsholt T, et al. Bacterial infection as a likely cause of adverse reactions to polyacrylamide hydrogel fillers in cosmetic surgery. Clin Infect Dis 2013;56:1438-44. [Crossref] [PubMed]
Cite this article as: Xie L, Wan H, Shao S, Qu W, Feng J, Gao Q, Sun J, Wu X. Granulomatous mastitis and pectoralis major muscle defect following polyacrylamide hydrogel injection: a case report and literature review. AME Case Rep 2024;8:46.