
Effective treatment of MET exon 14 skipping mutation-positive non-small cell lung cancer using capmatinib following serious maculopapular rash caused by two MET inhibitors: a case report
Highlight box
Key findings
• Re-administering mesenchymal-epithelial transition factor (MET) inhibitors, even in the second round, can effectively control treatment response and adverse events in MET exon 14 skipping mutation-positive non-small cell lung cancer (NSCLC) with recurrent severe maculopapular rash.
What is known and what is new?
• In a few cases, re-administration of MET inhibitors has demonstrated effectiveness in managing MET exon 14 skipping mutation-positive NSCLC with prior MET inhibitor-induced adverse events.
• In some MET exon 14 skipping mutation-positive NSCLC cases, a second round of MET inhibitor re-administration may continue to effectively manage therapeutic efficacy and adverse events.
What is the implication, and what should change now?
• Consideration of MET inhibitor re-administration after discontinuation due to ad-verse events can be among the treatment strategies for MET exon 14 skipping mutation-positive NSCLC.
Introduction
Background
Advanced-stage non-small cell lung cancer (NSCLC) is a highly lethal malignancy (1). However, the widespread adoption of cancer multi-gene panel testing and advancements in molecular targeted therapy have partially facilitated prolonged overall survival (OS) (2). In some cases, even with this approach, treatment-related adverse events (TRAEs), such as severe maculopapular rash, have been shown to potentially impede effective management of the condition.
Rationale and knowledge gap
Mesenchymal-epithelial transition factor (MET) exon 14 skipping mutation-positive NSCLC, which remains untreated with MET inhibitors, has a poorer prognosis compared with that of NSCLC without MET mutations (3). Therefore, evaluating the feasibility of using MET inhibitors following the occurrence of serious TRAEs is crucial for developing an effective treatment strategy. Despite limited reports on the re-administration of a MET inhibitor following the onset of serious TRAEs (4,5), the evidence supporting its effectiveness remains insufficient (1).
Objective
Herein, we present a case of successful management of grade 3 (G3) maculopapular rash, according to the Common Terminology Criteria for Adverse Events version 5.0, in a patient diagnosed with MET exon 14 skipping mutation-positive NSCLC. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-181/rc).
Case presentation
A never-smoker man in his 40s with a history of progressively worsening right chest and back pain was diagnosed with MET exon 14 skipping mutation-positive stage IVB (cT2aN1M1c) lung adenocarcinoma with multiple intrapulmonary, pleural, and bone metastases but without brain metastases (Table 1), exhibiting a programmed death-ligand 1 tumor proportion score of 1–4%. The patient developed progressive disease (PD) during fourth-line pembrolizumab treatment and was referred to our department. Physical examination revealed no abnormalities except diminished breath sounds in the right lower chest area.
Table 1
| Analytical results at different times | Sample | Genes | Isoform | Locus |
|---|---|---|---|---|
| Result before treatment | Tumor tissue | |||
| Gene fusions | MET-MET | MET-MET.M13M15.1 | chr7:116444708–chr7:116414935 | |
| Copy number variations | Not detected | Not detected | Not detected | |
| Result before third-line tepotinib treatment | Pleural effusion | |||
| Gene fusions | MET-MET | MET-MET.M13M15.1 | chr7:116444708–chr7:116414935 | |
| Copy number variations | FGFR3 | chr4: 1800932 | 0.27 |
†, Oncomine Precision Assay. MET, mesenchymal-epithelial transition factor; FGFR, fibroblast growth factor receptor.
During the health examination, an abnormality was identified in a chest photograph three months before the initial treatment. Subsequently, a diagnosis of MET exon 14 skipping mutation-positive NSCLC was confirmed through trans-bronchial biopsy. The treatment history is presented in Figure 1. Treatment was initiated with MET-inhibitory investigational drug (full dose), as first-line therapy; however, on day 11, pruritic macules and papules developed all over the body, excluding the hands, which were diagnosed as G3 maculopapular rashes. Dexamethasone (4 mg/d) was administered, and the skin rash was resolved. Subsequently, the dose of MET-inhibitory investigational drug was reduced to 83.3%. However, the malignant pleural effusion worsened, resulting in PD within 9 months. Second-line treatments included nivolumab, ipilimumab, carboplatin, and pemetrexed. The patient experienced PD owing to bone metastasis and worsening malignant pleural effusion. Companion diagnostic (CDx) analysis of malignant pleural fluid specimens revealed no specific secondary resistance (Table 1).
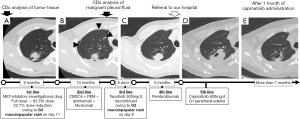
A combination of tepotinib and dexamethasone (4 mg/d) was administered as the third-line treatment but discontinued on day 8 owing to the re-development of the G3 maculopapular rash. For the fourth-line treatment, pembrolizumab was used, but PD occurred after two courses. Nonetheless, the patient opted to continue pembrolizumab treatment and was prescribed dexamethasone (4 mg/d) and oxycodone (30 mg/d) to alleviate nausea and pain.
After visiting our department, the patient received three additional courses of pembrolizumab based on personal preference. However, the treatment was discontinued because the malignant pleural effusion and pain worsened. In line with the personal preference for re-administering a MET inhibitor, capmatinib was utilized as the fifth-line therapy. Considering the history of TRAEs, the dexamethasone dosage was increased to 8 mg/d, and the initial daily dose of capmatinib was set at 600 mg. After 1 month of capmatinib treatment, chest computed tomography revealed rapid tumor regression and reduced symptoms. Consequently, dexamethasone and oxycodone were reduced to 4 and 5 mg/d, respectively, and no TRAEs were detected except for G1 peripheral edema. The performance status of the patient was reported to be 1, and his appetite and level of activities improved to approximately 70% of their pre-NSCLC levels. The patient could successfully resume work. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Key findings
We present, to our knowledge, the first documented case of a severe maculopapular rash associated with the MET-inhibitory investigational drug and tepotinib, which led to a switch to treatment with capmatinib and the successful alleviation of symptoms.
Comparison with similar research
Although the pathophysiology of maculopapular rash has not been fully elucidated, it is considered a hypersensitivity reaction and is reported in patients with rearranged during transfection (RET) fusion-positive NSCLC and prior exposure to immune checkpoint inhibitors used predominantly for their treatment (2). Skin rash (grade ≥3) has been reported in 0.6% of patients with RET fusion-positive NSCLC treated with selpercatinib (2). The combination of corticosteroids and a reduced selpercatinib dose successfully achieved a re-challenge rate of 86% in RET fusion-positive NSCLC (2).
The VISION study reported a case (0.7%) of maculopapular rash in MET-mutant NSCLC (6), whereas no such cases were reported in the GEOMETRY mono-1 study (7). In a recent real-world study by Paik et al., less than 20% of the patients discontinued treatment, and among them, 90% were attributed solely to PD (8). These data indicate a considerably low probability of treatment discontinuation caused by the appearance of skin rash in MET-mutant NSCLC. To our knowledge, no cases of repeated re-administration of MET inhibitors after resolving severe TRAEs have been reported.
Explanations of findings
In our case, the association of maculopapular rash, a grade ≥3 TRAE, with the MET-inhibitory investigational drug and tepotinib rather than capmatinib remains unclear; several reasons can be considered. A severe maculopapular rash following tepotinib, combined with dexamethasone, can be attributed to the prior usage of nivolumab and ipilimumab. As the one-step dose reduction dosage of tepotinib is a 50% dose reduction, administration of tepotinib without dose reduction may have also caused the maculopapular rash. In contrast, capmatinib was applied only after pembrolizumab treatment, a 25% dose reduction, and an increased dose of dexamethasone may have avoided serious TRAEs. Recent studies have reported alterations in metabolic pathways induced by CYP3A (9) and the ability of capmatinib and tepotinib to access lysosomes (10), but none of these features for the MET-inhibitory investigational drug; these differences may also have influenced TRAEs. In addition, the tumor progressed after the first-line MET inhibitor treatment but responded to capmatinib. The reason is also unclear but may be attributed to the anticancer drugs administered between the three MET inhibitors (11), as well as slight differences in the mechanism of action among these MET inhibitors (12). While these factors partially explain this fact, the lack of concrete evidence to explain the specific phenomenon is noticeable.
Strengths and limitations
In this case, MET-inhibitory investigational drug treatment resulted in the development of PD, and severe maculopapular rash reappeared despite the administration of a combination of tepotinib and dexamethasone, whereas capmatinib was attempted to be administered. There are no clinical guidelines that recommend re-administrating MET inhibitors after disease progression or when experiencing a grade ≥3 TRAE with MET inhibitors.
Conclusions
In situations with limited alternative treatment options, the cautious repeated re-administration of a different MET inhibitor may offer potential benefits to prolong OS for certain patients with a history of severe maculopapular rash caused by MET inhibitors. Further research and careful analyses are warranted to confirm this inference.
Acknowledgments
The authors thank the patient for allowing us to publish this case report.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-181/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-181/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-181/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cortot A, Le X, Smit E, et al. Safety of MET Tyrosine Kinase Inhibitors in Patients With MET Exon 14 Skipping Non-small Cell Lung Cancer: A Clinical Review. Clin Lung Cancer 2022;23:195-207. [Crossref] [PubMed]
- Illini O, Fabikan H, Swalduz A, et al. Real-world experience with capmatinib in MET exon 14-mutated non-small cell lung cancer (RECAP): a retrospective analysis from an early access program. Ther Adv Med Oncol 2022;14:17588359221103206. [Crossref] [PubMed]
- Tong JH, Yeung SF, Chan AW, et al. MET Amplification and Exon 14 Splice Site Mutation Define Unique Molecular Subgroups of Non-Small Cell Lung Carcinoma with Poor Prognosis. Clin Cancer Res 2016;22:3048-56. [Crossref] [PubMed]
- Kunimasa K, Kawamura T, Tamiya M, et al. Capmatinib successfully overcomes tepotinib-induced intolerable peripheral edema. Thorac Cancer 2021;12:3426-8. [Crossref] [PubMed]
- Hashiguchi MH, Sato T, Yamamoto H, et al. Successful Tepotinib Challenge After Capmatinib-Induced Interstitial Lung Disease in a Patient With Lung Adenocarcinoma Harboring MET Exon 14 Skipping Mutation: Case Report. JTO Clin Res Rep 2022;3:100271. [Crossref] [PubMed]
- Paik PK, Felip E, Veillon R, et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N Engl J Med 2020;383:931-43. [Crossref] [PubMed]
- Wolf J, Seto T, Han JY, et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:944-57. [Crossref] [PubMed]
- Paik PK, Goyal RK, Cai B, et al. Real-world outcomes in non-small-cell lung cancer patients with MET Exon 14 skipping mutation and brain metastases treated with capmatinib. Future Oncol 2023;19:217-28. [Crossref] [PubMed]
- Mathieu LN, Larkins E, Akinboro O, et al. FDA Approval Summary: Capmatinib and Tepotinib for the Treatment of Metastatic NSCLC Harboring MET Exon 14 Skipping Mutations or Alterations. Clin Cancer Res 2022;28:249-54. [Crossref] [PubMed]
- Berges N, Klug JH, Eicher A, et al. Differences in Sustained Cellular Effects of MET inhibitors Are Driven by Prolonged Target Engagement and Lysosomal Retention. Mol Pharmacol 2023;103:77-88. [Crossref] [PubMed]
- Kashizaki F, Tanaka A, Hattori S, et al. Dabrafenib-trametinib combination therapy re-challenge in advanced BRAFV600E-mutant non-small-cell lung cancer. Eur J Cancer 2021;143:31-2. [Crossref] [PubMed]
- Kashizaki F, Chen H, Miyasaka A, et al. Safety of Readministration of EGFR-TKI After Onset of Interstitial Lung Disease in Advanced EGFR-Mutated NSCLC: A Systematic Review and Meta-Analysis. Clin Lung Cancer 2024;25:e52-7. [Crossref] [PubMed]
Cite this article as: Kashizaki F, Okazaki S, Tsuchiya N, Chen H, Koizumi H, Takahashi K. Effective treatment of MET exon 14 skipping mutation-positive non-small cell lung cancer using capmatinib following serious maculopapular rash caused by two MET inhibitors: a case report. AME Case Rep 2024;8:42.


