
Complete response after treatment of breast cancer with isolated liver metastasis: a case report
Highlight box
Key findings
• Maintenance therapy may be the best option for patients with breast cancer whose liver metastases disappear after treatment. Moreover, in human epidermal growth factor receptor-2 (HER2)-positive metastatic breast cancer, patients with isolated liver metastases may be more likely to achieve a cure-like outcome.
What is known and what is new?
• A growing body of evidence supports the effectiveness of using anti-HER2 in conjunction with chemotherapy to treat breast cancer.
• Little is known about its impact on metastatic liver disease. Moreover, no studies have been conducted on managing the disappearance of liver metastases after drug treatment.
What is the implication, and what should change now?
• A cure-like outcome for breast cancer patients with isolated liver metastases may be possible with an aggressive treatment strategy. Maintenance therapy may be the most appropriate option for those who have undergone drug therapy resulting in the disappearance of liver metastases.
Introduction
In recent years, the incidence of breast cancer in women has surpassed lung cancer to become the most common cancer worldwide. It seriously affects the lives and health of women globally (1). About 15–20% of breast cancer patients express human epidermal growth factor receptor-2 (HER2) on the surface of cancer cells (2). This subtype of breast cancer is highly aggressive, develops rapidly, and often has a poor prognosis. Recurrent or metastatic HER2-positive breast cancer is considered incurable, and treatment efficacy is often not ideal (2). In particular, the median overall survival for HER2-positive metastatic breast cancer (MBC) is only 4–5 years, and the average survival rate of untreated patients with liver metastases is only 4–8 months (2-4). Also, breast cancer is prone to metastasis, while isolated liver metastasis is rare (5). Although a growing body of evidence supports the effectiveness of using anti-HER2 in conjunction with chemotherapy to treat breast cancer, little is known about its impact on metastatic liver disease. Furthermore, no studies have been conducted on how to handle cases where breast cancer liver metastases disappear after treatment. Here, we report on a patient with HER2-positive breast cancer with isolated liver metastases who achieved a complete pathological response of the primary lesion and the disappearance of liver metastases after drug treatment. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-104/rc).
Case presentation
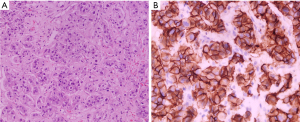
In May 2021, a 51-year-old postmenopausal Chinese woman presented to the Yongchuan Hospital of Chongqing Medical University with a painless irregular mass in her left breast with no medical history or co-morbidities. An ultrasound and mammogram were performed to evaluate the breast lesion, revealing two connected irregular masses in the lower inner quadrant of the breast, measuring 1.8 cm × 1.1 cm and 2.2 cm × 1.6 cm, respectively, with tiny calcifications inside and higher glandular density behind the nipple. No abnormalities were detected in the bilateral axillary lymph nodes. An abdominal computed tomography (CT) scan revealed a 4.6 cm × 3.0 cm mass-like low-density lesion in the liver’s S5 and S8 segments, but no evidence of metastasis was found in other examinations. Subsequently, suspicious lesions in the breast and liver were biopsied. The left breast biopsy revealed invasive carcinoma of the non-specific type, with a grade II score of 7, ER (−), PR (−), HER2 (3+), and ki67 about 30% (+). The liver biopsy showed breast cancer liver metastasis, with ER (−), PR (−), and HER2 (3+), consistent with the primary lesion (Figure 1).
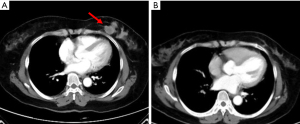
Given that this was a HER2-positive patient with advanced breast cancer, the patient was recommended to be treated with trastuzumab combined with pertuzumab and a paclitaxel-like chemotherapy agent. Finally, the patient was treated with nab-paclitaxel (260 mg/m2) with trastuzumab (first 8 mg/kg, subsequent 6 mg/kg) and pertuzumab (first 840 mg, subsequent 420 mg) for 6 cycles, followed by 11 cycles of dual-target therapy with trastuzumab (6 mg/kg) in combination with pertuzumab (420 mg/dose), for a total of 17 cycles, each cycle three weeks apart. The patient tolerated the treatment well without adverse events. After 6 cycles of chemotherapy combined with double-targeted treatment, the patient’s liver metastasis decreased from 4.6 cm × 3.0 cm before treatment to 0.8 cm × 0.5 cm (Figure S1). The mass in the inner lower quadrant of the breast disappeared upon CT imaging (Figure 2). Subsequently, after 17 cycles of treatment, the patient expressed a greater desire for surgery. Later, a preoperative examination including magnetic resonance imaging (MRI), CT, single-photon emission CT (SPECT), ultrasound, and ultrasonic contrast revealed the disappearance of the liver metastasis lesion. Meanwhile, no evidence of metastasis elsewhere was found (Figure 3). Preoperative breast MRI only exhibited mild patchy enhancement at the primary lesion without a definitive lesion being observed (Figure S2). The patient was unable to perform positron emission tomography (PET) to assess treatment response due to financial constraints.
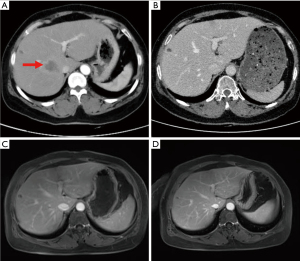
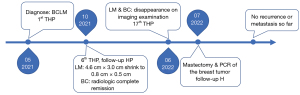
Considering the patient’s remarkable response to treatment and retrospective studies with extensive sample data and meta-analysis suggesting that resection of the primary tumor improves survival time in patients with stage IV breast cancer (6,7), we decided to proceed with resection of the primary breast cancer. The patient underwent a simple mastectomy of the left side, and the postoperative pathological examination revealed a complete pathology response of the tumor (Figure S3). As for the liver, where the metastatic lesions disappeared, the patient was recommended for follow-up and observation after a multidisciplinary discussion. The main reasons for this recommendation are that the metastatic liver lesions have disappeared and cannot be localized, the complete pathological response of the breast lesion, and the absence of drug resistance in the patient. Moreover, the optimal duration of maintenance anti-HER2 therapy for advanced breast cancer patients who have achieved complete remission remains unclear and needs to be balanced against treatment toxicity, logistical burden and cost (8). Here we recommend two options for patient follow-up: (I) continue dual-target maintenance therapy, which is more helpful in delaying disease progression; (II) single-target maintenance therapy, switching to dual-target intensive therapy once the disease progresses, increases the options available to patients after developing drug resistance. Finally, the patient was treated with trastuzumab (6 mg/kg) every three weeks as maintenance therapy and has continued to do well without disease during regular follow-ups until now (Figure 3). The timeline of important components of the case is in Figure 4.
All procedures performed in this study were in accordance with the ethical standards of the Yongchuan Hospital of Chongqing Medical University research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Although the incidence of breast cancer is high, the primary cause of mortality among breast cancer patients is tumor metastasis (9). Bone, lung, liver, and brain are the most frequent sites of distant breast cancer metastasis. Among them, the prognosis of liver metastasis is poorer, and the overall survival rate is lower than that of bone metastasis and lung metastasis (10,11). While approximately 5–10% of breast cancer patients have distant metastases at first diagnosis, liver metastases among them are relatively rare. Only 1.5% of patients with breast cancer have exclusively liver metastases, and isolated liver metastases are found in only 0.38% of breast cancer patients at initial diagnosis (5,12). In addition, significant tumor invasion, a high recurrence rate, and a poor prognosis are also associated with HER2 amplification or overexpression.
Combining chemotherapy with dual targets has enhanced the survival benefits of patients with HER2-positive MBC. In the CLEOPATRA trial, which followed untreated locally recurrent or MBC (LR/MBC) patients for over eight years, the experimental group receiving docetaxel with trastuzumab and pertuzumab therapy had a median progression-free survival (PFS) that was 6.1 months longer than the control group and a median overall survival that was 16.3 months longer than the control group. Moreover, the experimental group had an overall survival rate of 37% at 8 years (13). Similar efficacy data were reported in the PUFFIN trial, a Chinese bridge study to CLEOPATRA (14). Furthermore, in the PERUSE study, paclitaxel combined with trastuzumab and pertuzumab demonstrated a median PFS of 20.7 months in 1,436 HER2-positive LR/MBC patients, with one-third of the patients achieving a median PFS of 5.7 years. It was also established that paclitaxel is a viable alternative to docetaxel (15). Unfortunately, none of the experiments above specifically describe metastatic liver disease, and it remains unclear whether these findings can reasonably be applied to any metastatic site.
Our experience indicates that the efficacy of chemotherapy combined with dual-target therapy for breast cancer patients with isolated liver metastases is remarkable, promising a cure-like outcome and long-term patient survival. It is worth noting that recent long-term follow-up studies on anti-HER2 therapy have revealed that a small portion of patients with HER2-positive MBC may achieve a long-term response similar to a cure, challenging the traditional belief that advanced HER2-positive breast cancer is incurable (16). Additionally, the patient is satisfied with the therapy she received, with no signs of recurrence or metastasis observed during regular follow-ups.
Although some studies suggest that local treatment of liver metastases may improve long-term survival in some patients (17,18), the available evidence is based on highly selected patient series and lacks randomized data. Moreover, there is no data on the best technique for local treatment for individual patients (8). The National Comprehensive Cancer Network (NCCN) guidelines do not recommend surgical treatment for MBC due to a lack of evidence supporting it (19,20). Similarly, the Chinese Anti-Cancer Association Guidelines and Standards for Breast Cancer Diagnosis and Treatment also note that the value of local therapy in patients with stage IV breast cancer is unclear (21). Ongoing phase III randomized controlled trials like the NRG-BR002 trial (NCT02364557), STEREO-SEIN (NCT02089100), and OLIGOMA (NCT04495309) aim to determine whether treatment of distant metastases can provide benefits to patients with MBC and may help guide future treatment decisions.
As for managing the primary site in advanced breast cancer, most retrospective clinical studies have concluded that overall survival is beneficial after localized surgery of the primary lesion in de novo stage IV breast cancer. Nevertheless, according to the recently published results of the JCOG1017 phase III randomized controlled clinical trial by the Japanese Study Group, resection of the primary lesion did not significantly improve the overall survival of patients with de novo stage IV breast cancer. However, resection of the primary lesion improves survival in patients with single-organ metastases while providing a definite benefit in localized disease control.
Conclusions
The local treatment of metastases in patients with liver metastases from breast cancer is still very controversial. This case suggests that maintenance therapy may be the best option for patients whose breast cancer liver metastases have disappeared by medication. Also, we can infer that in HER2-positive MBC, patients with isolated liver metastases are more likely to achieve a cure-like outcome. However, more cases and follow-up data are needed to validate these views.
Acknowledgments
The authors thank the members of their department for their assistance.
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-104/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-104/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-104/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Bredin P, Walshe JM, Denduluri N. Systemic therapy for metastatic HER2-positive breast cancer. Semin Oncol 2020;47:259-69. [Crossref] [PubMed]
- Zuo Q, Park NH, Lee JK, et al. Liver Metastatic Breast Cancer: Epidemiology, Dietary Interventions, and Related Metabolism. Nutrients 2022;14:2376. [Crossref] [PubMed]
- Deluche E, Antoine A, Bachelot T, et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008-2016. Eur J Cancer 2020;129:60-70. [Crossref] [PubMed]
- Zhao HY, Gong Y, Ye FG, et al. Incidence and prognostic factors of patients with synchronous liver metastases upon initial diagnosis of breast cancer: a population-based study. Cancer Manag Res 2018;10:5937-50. [Crossref] [PubMed]
- Vohra NA, Brinkley J, Kachare S, et al. Primary tumor resection in metastatic breast cancer: A propensity-matched analysis, 1988-2011 SEER data base. Breast J 2018;24:549-54. [Crossref] [PubMed]
- Harris E, Barry M, Kell MR. Meta-analysis to determine if surgical resection of the primary tumour in the setting of stage IV breast cancer impacts on survival. Ann Surg Oncol 2013;20:2828-34. [Crossref] [PubMed]
- Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020;31:1623-49. [Crossref] [PubMed]
- Wang R, Zhu Y, Liu X, et al. The Clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer 2019;19:1091. [Crossref] [PubMed]
- Xie Y, Ma J, Xia X, et al. Prognosis and Treatment of Metastatic Breast Cancer From A Real-World Scenario in China: A Retrospective Cohort Study. Cancer Control 2022;29:10732748221130568. [Crossref] [PubMed]
- La Verde N, Collovà E, Blasi L, et al. Overall Survival in Metastatic Breast Cancer Patients in the Third Millennium: Results of the COSMO Study. Clin Breast Cancer 2021;21:e489-96. [Crossref] [PubMed]
- Cardoso F, Spence D, Mertz S, et al. Global analysis of advanced/metastatic breast cancer: Decade report (2005-2015). Breast 2018;39:131-8. [Crossref] [PubMed]
- Swain SM, Miles D, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol 2020;21:519-30. [Crossref] [PubMed]
- Xu B, Li W, Zhang Q, et al. Pertuzumab, trastuzumab, and docetaxel for Chinese patients with previously untreated HER2-positive locally recurrent or metastatic breast cancer (PUFFIN): a phase III, randomized, double-blind, placebo-controlled study. Breast Cancer Res Treat 2020;182:689-97. [Crossref] [PubMed]
- Miles D, Ciruelos E, Schneeweiss A, et al. Final results from the PERUSE study of first-line pertuzumab plus trastuzumab plus a taxane for HER2-positive locally recurrent or metastatic breast cancer, with a multivariable approach to guide prognostication. Ann Oncol 2021;32:1245-55. [Crossref] [PubMed]
- Tarantino P, Curigliano G, Parsons HA, et al. Aiming at a Tailored Cure for ERBB2-Positive Metastatic Breast Cancer: A Review. JAMA Oncol 2022;8:629-35. [Crossref] [PubMed]
- Chun YS, Mizuno T, Cloyd JM, et al. Hepatic resection for breast cancer liver metastases: Impact of intrinsic subtypes. Eur J Surg Oncol 2020;46:1588-95. [Crossref] [PubMed]
- Galiandro F, Agnes S, Moschetta G, et al. Prognostic Factors in Patients with Breast Cancer Liver Metastases Undergoing Liver Resection: Systematic Review and Meta-Analysis. Cancers (Basel) 2022;14:1691. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Abraham J, et al. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020;18:452-78. [Crossref] [PubMed]
- Gradishar WJ, Moran MS, Abraham J, et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:691-722. [Crossref] [PubMed]
- Chinese Anti-Cancer Association Breast Cancer Professional Committee. Chinese Anti-Cancer Association Breast Cancer Diagnosis and Treatment Guidelines and Specifications (2021 Edition). China Cancer Journal 2021;31:954-1040.
Cite this article as: Zhang T, Liu Y, Yang L, Tian T. Complete response after treatment of breast cancer with isolated liver metastasis: a case report. AME Case Rep 2024;8:28.


