Successful percutaneous catheter-directed treatment of high-risk pulmonary embolism: a case report
Highlight box
Key findings
• Percutaneous catheter-directed treatments represent an effective and safe alternative therapy for PE
What is known and what is new?
• The rate of in-hospital mortality in hemodynamically unstable patients with PE is high; in this setting, ESC guidelines recommend anticoagulation therapy and hemodynamic support.
• Nowadays several catheter-directed treatments for PE are available; in our experience, mechanical thrombectomy with the Inari Flow system is safe and effective.
What is the implication, and what should change now?
• Results of randomized clinical trials are expected to demonstrate the superiority of percutaneous device treatments of PE over medical therapy, to change guidelines recommendations.
Introduction
Venous thromboembolism (VTE), clinically presented as deep vein thrombosis (DVT) or Pulmonary embolism (PE), is globally the third most frequent acute cardiovascular syndrome. The annual incidence rate of VTE ranges between 75 and 269 cases per 100,000 persons (1). Using the raw NHS Outcomes Framework data and population statistics for England in 2018, Roberts and colleagues calculate a crude annual VTE mortality rate of 21.7% per 100,000 (2). Between 2000 and 2015, age-standardized annual PE-related mortality rates decreased linearly from 12.8% to 6.5% deaths per 100,000 population without substantial sex-specific differences (3).
PE therapy in the acute phase is based on hemodynamic and ventilatory support and the initiation of anticoagulation therapy. In the case of high-risk PE, when the 30-day mortality risk is >10%, European Society of Cardiology (ESC) guidelines recommend systemic thrombolytic therapy; if thrombolysis is contraindicated or has failed, surgical pulmonary embolectomy is recommended (4).
Due to the fast development of percutaneous systems, nowadays, many catheter-directed treatments for PE are available, but robust data supporting their use are lacking. Guidelines include the possibility of using transcatheter therapy in high-risk patients in whom thrombolysis is contraindicated or has failed (IIa class of recommendation). We report a case of a 55 years-old man with massive PE treated with a novel percutaneous catheter system that performed mechanical thrombectomy. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-71/rc).
Case presentation
A 55 years-old man, a former smoker, affected by arterial hypertension, was admitted to our Emergency Department with significant dyspnea. On hospital admission, his vitals were: blood pressure 90/60 mmHg, heart rate 110 beats per minute, 24 breaths per minute, and temperature 36 ℃. Electrocardiogram showed sinus tachycardia with right bundle branch block; laboratory tests revealed a high D-dimer level (7,082 ng/mL), Fibrinogen 422 mg/dL, Ultrasensitive Troponin I 1,098.2 ng/L; the echo-fast demonstrated right ventricular dilatation [right ventricular (RV) > left ventricular (LV)], with reduced contractility (TAPSE 10 mm) and severe tricuspid regurgitation, with 75 mmHg pulmonary artery (PA) systolic pressure.
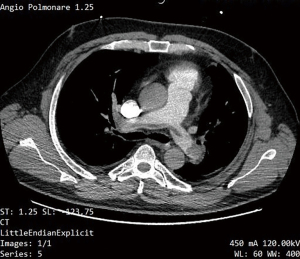
Computed tomography (CT) pulmonary angiography showed simultaneous filling defects of the right and left PA: a diagnosis of massive PE was made (Figure 1). During CT examination, patient’s blood pressure worsened to 75/50 mmHg. The Simplified PE Severity Index (sPESI) score was 3, which classified the patient as at high risk of mortality.
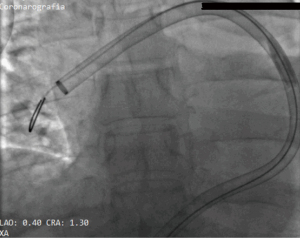
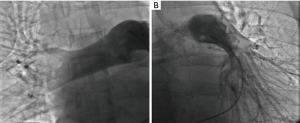
Due to the rapid haemodynamic deterioration with a clear hemodynamic instability, the sPESI score, and the availability of a dedicated percutaneous aspiration catheter in our Cath Lab, we decided to treat the patient with mechanical thrombectomy. After placing a 24 French (Fr) flex introducer sheath into the right common femoral vein, a 5-Fr angled Pigtail catheter was advanced over a 0.035-inch guidewire to reach the pulmonary trunk; so a pulmonary angiography was performed: it confirmed a massive embolism, especially of the right PA, with angiographic Miller Index of 23 (Figure 2). Thus, we utilized the FlowTriever 2 system (Inari Medical, Basel, Switzerland) which is a series of over-the-wire catheters system (that can treat vessels from 6 to 16 cm in diameter) able to remove large clots through both mechanical and aspiration mechanisms. The new 2nd FlowTriever aspiration catheter is characterized by a laser cut element with proximal open cell design for improved clot clearance and a novel disk shape planned to disrupt thrombus, improving effectiveness of aspiration.
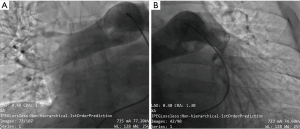
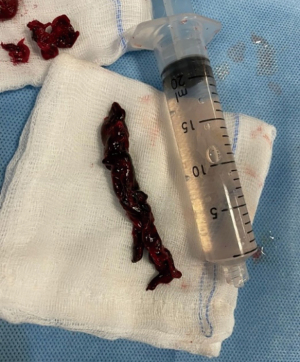
We advanced an Amplatzer Stiff guide in the right PA, but the 24 Fr aspiration catheter did not reach the artery due to poor support; thus, we used a Lunderquist Extra-Stiff guide wire (Cookmedical, Bloomington, USA) to advance successfully the FlowTriever 2 aspiration catheter in the main right PA (Figure 3); the main PA pressure of 28 mmHg was registered. At this point, the catheter captured a huge thrombus of about 15 cm in length extended from the right PA to the left PA, that was successfully aspirated and brought out from the catheter (Figure 4). Final angiography demonstrated angiographic Miller Index of 6 (Figure 5) and mean PA pressure was 16 mmHg.
At the end of the procedure the patient was hemodynamically stable and asymptomatic. He was then admitted to Intensive Coronary Care Unit and oral anticoagulation therapy with Rivaroxaban 15 mg bis in die was started. Ten days after the procedure, CT pulmonary angiogram showed complete resolution of filling defects in the principal pulmonary arteries. Patient was discharged after 12 days of hospitalization, due to limited prompt availability of the second-level imaging tests to investigate the origin of thrombus.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Despite the trend in the reduction of mortality, PE represents a relevant medical problem because of its high incidence. The rate of in-hospital mortality in hemodynamically unstable patients is high and reported to reach nearly 32% (5). Patients with PE are classified according to the short-term risk of mortality, that is based on clinical characteristics, laboratory tests and imaging findings: high-risk PE, defined by a 30-days risk of mortality >10%, accounts for less than 10% of PE cases and represents a life-threatening medical emergency, requiring immediate reperfusion treatment to prevent death (6); intermediate-risk PE patients are defined as hemodynamically stable, but they should be rigorously monitored, because some can deteriorate and eventually require reperfusion therapy (7).
Although ESC guidelines recommend systemic thrombolytic therapy for high-risk PE and immediate anticoagulation for intermediate-risk (4), only a minority of patients receive systemic thrombolysis, due to comorbidities or to the 10% risk of major hemorrhagic side effects; further this therapy does not work in 8% of people who receive it (7).
Actually, percutaneous catheter-directed treatment is recommended only in high-risk patients in whom thrombolysis is contraindicated or has failed and surgical embolectomy is not available (IIa class of recommendation) and in intermediate-risk patients with hemodynamic deterioration, because its benefits over medical or surgical therapy are still unclear. However, a clinical consensus statement by the ESC Working Group on pulmonary circulation and right ventricular function and the European Association of Percutaneous Cardiovascular Interventions has just been published and it proposed algorithm and timelines of catheter-directed therapies, demonstrating a growing clinical and scientific interest in this novel field (8).
Nowadays several percutaneous systems are available, based on catheter-directed thrombolysis and catheter mechanical embolectomy with thrombus maceration, rheolytic and rotational thrombectomy (Table 1) (9), but no one has large data that support its routine use.
Table 1
| Device | Mechanism | Technical consideration | Studies |
|---|---|---|---|
| Ekosonic | Ultrasound assisted thrombolysis | 5 Fr catheter | ULTIMA 2014 (10), SEATTLE II 2015 (11), OPTALYSE PE 2018 (12), SUNSET 2021 (13) |
| Unifuse | Catheter-directed thrombolysis | 4–5 Fr catheter | PERFECT trial 2015 (14), SUNSET 2021 (13) |
| Cragg McNamara | Catheter-directed thrombolysis | 4–5 Fr catheter | PERFECT trial 2015 (14), SUNSET 2021 (13) |
| Angio-Vac | Large lumen aspiration tube with the system of veno—venous by-pass | 26 Fr access for inflow and 16–20 Fr access for outflow | Donaldson et al. 2015 (15), Al-Hakim et al. 2016 (16) |
| AngioJet | Rheolytic thrombectomy with optional thrombolysis | 6–8 Fr catheters attached to a drive unit/pump for thrombectomy and/or infusing TPA | – |
| Indigo | Mechanical thrombectomy—aspiration with maceration | 8/12 Fr catheters with a suction pump/engine | EXTRACT PE 2021 (17) |
| FlowTriever | Mechanical thrombectomy—suction and maceration | 20/24 Fr aspiration large bore catheters. Access through the femoral or jugular vein | FLARE 2018 (18), Wible et al. 2019 (19), Yasin et al. 2020 (20) |
TPA, tissue plasminogen activator.
A recent systematic review of Harvey and colleagues revealed that there are no randomized clinical trials of any catheter-directed treatments in people with high-risk or intermediate risk PE, but the initial results from the prospective multicenter registry Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT) are encouraging, resulting in the improvement of clinical outcome and in the reduction of major bleedings with the use of catheter-directed thrombolysis (14). Nevertheless, one study reported a 10% of incidence of major extracranial haemorrhage requiring transfusion (11), thus based on current data, it is still unclear if these devices are better than systemic thrombolysis.
In our case, we performed a mechanical thrombectomy with the FlowTriever system that sucked and macerated the thrombus through a large bore catheter advanced in the PA. In a single-centre retrospective study of 46 patients that included both massive and sub-massive central PE with right heart strain, patients treated with the FlowTriever device reported significant reduction of mean pulmonary arterial pressure (MPAP) and oxygen requirements (19). In another small retrospective case study including 7 patients with intermediate-risk PE and 1 at high risk, the FlowTriever system demonstrated initial positive clinical experience and safety, favouring its use in the setting of acute PE, especially when thrombolysis is contraindicated (20).
Conclusions
We reported an early experience, in an Italian tertiary centre, of a high-risk patient treated with Inari Flow system, resulting in a fast improvement of vital signs and instant resolution of patient’ symptoms, without complications. Results of nine randomized clinical trials (8) are expected to demonstrate the superiority of percutaneous device treatments of PE over medical therapy, but based on our experience, percutaneous mechanical thrombectomy is an effective and safe alternative treatment for patients with PE at high and intermediate risk.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-71/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-22-71/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-71/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to the global disease burden. J Thromb Haemost 2014;12:1580-90. [Crossref] [PubMed]
- Roberts LN, Whyte MB, Arya R. Pulmonary embolism mortality trends in the European region-too good to be true? Lancet Respir Med 2020;8:e2. [Crossref] [PubMed]
- Barco S, Mahmoudpour SH, Valerio L, et al. Trends in mortality related to pulmonary embolism in the European Region, 2000-15: analysis of vital registration data from the WHO Mortality Database. Lancet Respir Med 2020;8:277-87. [Crossref] [PubMed]
- Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020;41:543-603. [Crossref] [PubMed]
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011;123:1788-830. [Crossref] [PubMed]
- Harvey JJ, Huang S, Uberoi R. Catheter-directed therapies for the treatment of high risk (massive) and intermediate risk (submassive) acute pulmonary embolism. Cochrane Database Syst Rev 2022;8:CD013083. [Crossref] [PubMed]
- Liu J, Liu Y, Zhang F, et al. Short-term prognostic value of clinical data in hospitalized patients with intermediate-risk acute pulmonary embolism. BMC Cardiovasc Disord 2022;22:335. [Crossref] [PubMed]
- Pruszczyk P, Klok FA, Kucher N, et al. Percutaneous treatment options for acute pulmonary embolism: a clinical consensus statement by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function and the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention 2022;18:e623-38. [Crossref] [PubMed]
- Moore K, Kunin J, Alnijoumi M, et al. Current Endovascular Treatment Options in Acute Pulmonary Embolism. J Clin Imaging Sci 2021;11:5. [Crossref] [PubMed]
- Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014;129:479-86. [Crossref] [PubMed]
- Piazza G, Hohlfelder B, Jaff MR, et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc Interv 2015;8:1382-92. [Crossref] [PubMed]
- Tapson VF, Sterling K, Jones N, et al. A Randomized Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Risk Pulmonary Embolism: The OPTALYSE PE Trial. JACC Cardiovasc Interv 2018;11:1401-10. [Crossref] [PubMed]
- Avgerinos ED, Jaber W, Lacomis J, et al. Randomized Trial Comparing Standard Versus Ultrasound-Assisted Thrombolysis for Submassive Pulmonary Embolism: The SUNSET sPE Trial. JACC Cardiovasc Interv 2021;14:1364-73. [Crossref] [PubMed]
- Kuo WT, Banerjee A, Kim PS, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): Initial Results From a Prospective Multicenter Registry. Chest 2015;148:667-73. [Crossref] [PubMed]
- Donaldson CW, Baker JN, Narayan RL, et al. Thrombectomy using suction filtration and veno-venous bypass: single center experience with a novel device. Catheter Cardiovasc Interv 2015;86:E81-7. [Crossref] [PubMed]
- Al-Hakim R, Park J, Bansal A, et al. Early Experience with AngioVac Aspiration in the Pulmonary Arteries. J Vasc Interv Radiol 2016;27:730-4. [Crossref] [PubMed]
- Sista AK, Horowitz JM, Tapson VF, et al. Indigo Aspiration System for Treatment of Pulmonary Embolism: Results of the EXTRACT-PE Trial. JACC Cardiovasc Interv 2021;14:319-29. [Crossref] [PubMed]
- Tu T, Toma C, Tapson VF, et al. A Prospective, Single-Arm, Multicenter Trial of Catheter-Directed Mechanical Thrombectomy for Intermediate-Risk Acute Pulmonary Embolism: The FLARE Study. JACC Cardiovasc Interv 2019;12:859-69. [Crossref] [PubMed]
- Wible BC, Buckley JR, Cho KH, et al. Safety and Efficacy of Acute Pulmonary Embolism Treated via Large-Bore Aspiration Mechanical Thrombectomy Using the Inari FlowTriever Device. J Vasc Interv Radiol 2019;30:1370-5. [Crossref] [PubMed]
- Yasin JT, Davis R, Saemi A, et al. Technical efficiency, short-term clinical results and safety of a large-bore aspiration catheter in acute pulmonary embolism - A retrospective case study. Lung India 2020;37:485-90. [Crossref] [PubMed]
Cite this article as: Russo D, Massaro G, Sangiorgi GM. Successful percutaneous catheter-directed treatment of high-risk pulmonary embolism: a case report. AME Case Rep 2023;7:5.