
Cutaneous squamous cell carcinoma with secondary parotid metastasis: a case report
Introduction
Squamous cell carcinoma (SCC) has been one of the commonest non-melanoma skin malignancies. Approximately 80–90% of cutaneous squamous cell carcinoma (cSCC) originate in the head and neck region (1), from which 1–3% have been found metastasizing to parotid or peri-parotid lymph nodes (2). Risk factors of parotid metastases from cSCC include primary tumor size >2 cm, tumor thickness >4 mm, incomplete excision margin (<4 mm), recurrent tumor, tumor near the parotid gland (ear, temple, forehead or anterior scalp), high grade or desmoplastic lesion, lymphovascular or perineural invasion, advanced age and immunocompromised host (3). With presence of secondary parotid metastasis, synchronous spread to the adjacent level II–V nodes occurs in 15% to 30% of cases (4).
Since the extent of parotid and neck nodal involvement has imposed a significant impact on the survival of patients with cSCC (5), early diagnosis and treatment are essential. However, in the absence of clinical or imaging findings in the parotid gland, the risk of occult metastases is not high enough to warrant an elective parotidectomy in many patients with cSCC. On contrary, total parotidectomy is indicated in the event of parotid metastasis from cSCC (6).
We report a case of metastatic left cheek cSCC to parotid gland that was surgically treated with total parotidectomy, modified radical neck dissection and soft tissue reconstruction with free anterolateral thigh fasciocutaneous flap and adjuvant radiotherapy with satisfactory outcomes. We present the following article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-64/rc).
Case presentation
This was an 85-year-old gentleman, a retired lumberjack, who presented with left cheek painless lesion for one year. The lesion appeared to be dark and round, which was progressively increasing in size. Otherwise, there was no skin ulceration, bleeding, discharge, itchiness, or neck swelling. In addition, he also did not have constitutional symptoms such as lethargy, unintentional weight loss or loss of appetite. He had multiple naevi present on his face and body, with prior history of right temporal SCC that was resected with clear margin 5 years ago. There was no family history of malignancy. He did not have social habits of alcohol consumption and tobacco use. His background medical illnesses included type 2 diabetes mellitus, hypertension, dyslipidemia, and benign prostatic hyperplasia. There was no other significant past surgical history. He was otherwise an active elderly gentleman who had independent activity of daily living status.
Clinical examination revealed a hyperpigmented dome-shape lesion located at left cheek, measuring approximately 1.7 cm × 1.4 cm. There was no associated ulceration, bleeding, discharge, satellite lesion, cervical or supraclavicular lymph nodes. Multiple naevi of varying sizes distributed over the face and neck. He then had wide local excision of left cheek lesion performed with a margin of 5 mm. The wound defect was closed with a modified rhomboid local flap. Histopathological result revealed moderately differentiated SCC with clear margin.
Four months after the primary surgery, he developed a new painless swelling at left angle of mandible. The swelling had progressively enlarged and associated with skin ulceration and whitish discharge. Otherwise, there was no bleeding, paresthesia, facial asymmetry, hearing impairment or constitutional symptoms. The swelling did not cause difficulty of mastication or swallowing.
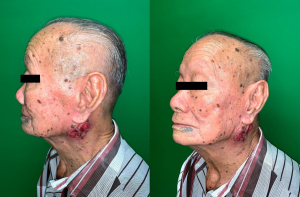
On clinical assessment, there was an ulcerative mass of irregular border and shape, lobulated surface, measuring 6 cm × 4 cm, located at left angle of mandible, extending to left mastoid region. The edge of the ulcer appeared to be everted and the base was filled with tumor tissues. There was no cervical or supraclavicular lymphadenopathy. Facial, lingual, and hypoglossal nerve functions were intact. There was a well-healed mature surgical scar of previous modified rhomboid flap surgery at left cheek (Figure 1). Systemic examination was unremarkable.
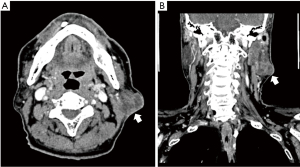
Fine needle aspiration cytology (FNAC) was performed in which the cytopathological results confirmed the finding of well-differentiated SCC. Contrast enhanced computer tomography scan of head and neck demonstrated an ill-defined, enhancing, lobulated, heterogenous, hypodense lesion at left parotid gland, measuring 2.5 cm × 3.2 cm × 2.5 cm, with infiltration into left sternocleidomastoid muscle posteriorly, associated with multiple subcentimeters left parotid periglandular, submental, submandibular and cervical lymph nodes enlargement (Figure 2). There was no evidence of invasion into the ipsilateral carotid sheath contents. Staging CT scan of thorax, abdomen and pelvis did not show any evidence of distant metastasis.
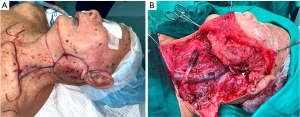
Subsequently, he underwent left total parotidectomy, ipsilateral modified radical neck dissection (type II), and soft tissue coverage with right free anterolateral thigh fasciocutaneous flap. Intra-operatively, the ulcerative mass was resected with a margin of 2 cm, including left ear lobule (Figure 3A). Facial nerve and its rami were identified and preserved during parotidectomy (Figure 3B). In addition, great auricular nerve and external jugular vein were both sacrificed. Upon completion of facial nerve dissection, both superficial and deep lobe of left parotid glands were removed. During ipsilateral modified radical neck dissection, left sternocleidomastoid muscle, level I to level V cervical lymph nodes and left submandibular gland were removed, while preserving spinal accessory nerve, carotid sheath contents (internal jugular vein, carotid artery and vagus nerve), phrenic nerve, hypoglossal nerves and transverse cervical vessels (Figure 3B). Specimens of wide local excision, total parotidectomy and modified radical neck dissection were tagged and sent for histopathological assessment. The wound defect was reconstructed with right free anterolateral thigh fasciocutaneous flap. Right thigh donor wound was closed primarily.
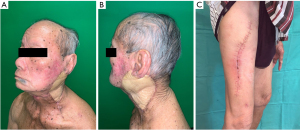
Post-operatively, he led an uneventful recovery. Histopathological result confirmed the finding of moderately differentiated SCC with superficial parotid lobe involvement. Otherwise, there was no evidence of lymphovascular invasion, perineural invasion or lymph node involvement (0 out of 28 lymph nodes). Deep lobe of parotid gland was free of malignancy. Both donor and recipient wounds had healed well and patient felt satisfied with the outcomes (Figure 4). He was subsequently subjected to adjuvant radiotherapy. At one year of follow-up, there was no recurrence or distant metastasis.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Nodal metastases to parotid gland from cSCC have been by far the commonest secondary parotid metastasis, accounting for 79% of all parotid metastases (7). Previous evidence has shown that approximately 15% of advanced (≥T2) cSCC have been found to have regional lymph nodes metastasis in parotid gland or neck (5). The frequency of location for primary cutaneous SCC in descending order is: ear/preauricular (about 39%), temple (19%), cheek (10%), scalp (10%), neck (7%), forehead (4%), lips (3%), and periorbital skin (2%) (8). Czerwonka et al. have shown that up to thirty percent of patients with cSCC have presented with parotid metastases upon diagnosis of cutaneous primary, while 70% have developed nodal metastases following management of their cutaneous primary (8). Risk factors of parotid metastasis from cutaneous SCC include primary tumor size >2 cm, tumor thickness >4 mm, incomplete excision margin (<4 mm), recurrent tumor, tumor near the parotid gland (ear, temple, forehead or anterior scalp), high grade or desmoplastic lesion, lymphovascular or perineural invasion, advanced age and immunocompromised host (3). In the case of this patient, a known primary has been evident – previously resected cSCC of left cheek, which has been predisposed by risk factors such as recurrent tumour, high-risk tumour location at pre-auricular area, and advanced age. Furthermore, histopathological confirmation of keratinizing SCC from the total parotidectomy specimen has further supported the diagnosis of secondary parotid metastases from cSCC (9). Both clinical examination and staging CT scan has not revealed any suspicious lesion suggestive of a primary arising from other supraclavicular (scalp, oropharynx, oral cavity, nasopharynx, or ear canal) or infraclavicular origin (lung or esophagus) (10). Metastatic parotid SCC of unknown primary has been defined as metastatic lesion without an identifiable source of primary tumour after performing an exhaustive clinical, radiographic, and surgical evaluation, and absence of typical histological characteristics of primary parotid SCC (11). The diagnosis of primary parotid SCC necessitates exclusion of metastasis from an extra-glandular primary and a typical histological characteristic—squamous metaplasia or dysplasia of salivary duct epithelium with infiltrative growth (10). Both are unlikely in the present case as there is an identifiable primary to the metastatic lesion.
The parotid gland has been the first draining site for cutaneous malignancies on the cheek, pinna, forehead, and temple (4). Anatomic studies have reported a range of 3–19 lymph nodes in the superficial lobe and 0–9 in the deep lobe (12-14). Secondary metastasis to parotid gland from cSCC can occur via lymphatic spread or direct invasion. The pattern of lymphatic spread for metastatic cSCC of head and neck has been found progressing from the first echelon lymph nodes within the superficial lobe of parotid gland to second echelon lymph nodes within the deep lobe of parotid gland and neck (6). Among patients who have been diagnosed with metastatic cSCC to superficial lobe of parotid gland, one-fourth are demonstrated to have parotid deep lobe metastases (6). The superficial and deep lobe specimens from total parotidectomy among patients with metastatic cSCC have been shown to harbor a mean positive lymph node of 1.7 and 0.4 out of 5.9 and 1.8 mean total lymph nodes, respectively (6). Data from Mayo Clinic have revealed cSCC with parotid deep lobe metastasis as a significant risk factor of distant metastatic disease, disease recurrence, death from disease, and overall survival (6). As such, total parotidectomy is recommended for patients with metastatic cSCC to parotid gland. Conversely, there is no role of elective parotidectomy in cSCC of head and neck with absence of metastatic disease to parotid gland, even in the advanced or high-risk disease (15). Based on the evidence detailed above, left nerve-sparing total parotidectomy is indicated in the present case in view of radiological and pathological evidence of left parotid metastasis pre-operatively.
Approximately 16.4% (28/171) (4) to 31% (13/42) (6) of patients with secondary parotid metastases from cutaneous SCC have been demonstrated to harbor concomitant cervical lymph node metastases. The frequency of cervical nodal positivity in descending orders is: level II (80%), level III (55%), level IV (40%), level V (35%) and level I (25%) (4). The incidence of occult neck metastases in patients with metastatic cSCC to parotid without clinical or radiological neck disease has been reported in the range of 15% to 44% (16-22). Occult nodal metastases predominantly occur at level II (89%), followed by level III (32%), level I (11%), level V (5%) and level IV (0%) (4). As for any head and neck cSCC, patients with concomitant parotid and cervical nodal metastases should be treated with ipsilateral comprehensive therapeutic neck dissection (23). Patients who have metastatic cSCC with a clinically negative neck nodes are recommended to undergo selective neck dissection to include levels I–III for facial primaries, levels II–III for anterior scalp and external ear primaries, and levels II–V for posterior scalp and neck primaries (23). In the presence of radiological neck disease and parotid metastases in this patient, modified radical neck dissection has been performed to remove all cervical lymph nodes from level I to level V. In addition, the neck dissection also has removed the left sternocleidomastoid muscle, which has been shown to be infiltrated by left parotid metastatic mass radiologically. Final histopathological result of the modified radical neck dissection specimen has shown no metastatic involvement of cervical lymph nodes (0 out of a total of 28 lymph nodes removed) and left sternocleidomastoid muscle.
Metastatic disease to the parotid gland often represents a biologically aggressive tumor that frequently possesses other pathologically aggressive features, including lymphovascular space and perineural invasion, and a propensity for distant spread. Melo and colleagues have demonstrated that locally advanced or relapsed cSCC from head and neck region with parotid metastasis are associated with parotid extracapsular spread (62.2%; 23/37), neck metastases (51.4%; 19/37), lymph node extracapsular spread (37.8%; 14/37), perineural invasion (37.8%; 14/37), and lymphovascular invasion (32.4%; 12/37). Furthermore, the same cohort has been found to have worse overall survival rate at 3, 5 and 10 years, comparing to cSCC without parotid metastases group (58% vs. 78%, 46% vs. 72% and 13% vs. 72%, P=0.0283) (24). In a study consisting of 42 patients with cSCC metastasizing to parotid glands, 5-year local (any parotid-area recurrence), and locoregional (any parotid-area and neck recurrence) recurrence rate post total parotidectomy and ipsilateral neck dissection in cohorts with and without deep lobe parotid metastases have been reported to be 11.1% (2/26) versus 11.0% (1/9), and 22% (2/9) versus 12% (3/26), respectively (6). The distant metastasis-free survival rate at 5 years is 36% with deep lobe parotid metastasis versus 76% without deep lobe parotid metastasis (6). The high recurrence rate associated with increased mortality therefore justify a more aggressive surgical approach consisting of total parotidectomy and ipsilateral neck dissection in patients who have developed secondary parotid metastases from cSCC. Addition of adjuvant radiotherapy to surgery has been shown to further improve locoregional control (20). The present case is therefore subjected to adjuvant radiotherapy.
Conclusions
Although rare, metastatic cSCC to parotid gland represents a unique group of locally advanced cSCC. Multimodal treatment approach consisting of total parotidectomy, ipsilateral neck dissection and adjuvant radiotherapy has been shown to improve the locoregional control of the disease and limits the propensity to distant metastasis. The present case has illustrated a good example of multimodal management regime in secondary parotid metastasis from cSCC.
Acknowledgments
We would like to thank surgical specialists and residents who were involved in the surgery.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-64/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-64/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med 2001;344:975-83. [Crossref] [PubMed]
- Hinerman RW, Indelicato DJ, Amdur RJ, et al. Cutaneous squamous cell carcinoma metastatic to parotid-area lymph nodes. Laryngoscope 2008;118:1989-96. [Crossref] [PubMed]
- Veness MJ, Palme CE, Smith M, et al. Cutaneous head and neck squamous cell carcinoma metastatic to cervical lymph nodes (nonparotid): a better outcome with surgery and adjuvant radiotherapy. Laryngoscope 2003;113:1827-33. [Crossref] [PubMed]
- Vauterin TJ, Veness MJ, Morgan GJ, et al. Patterns of lymph node spread of cutaneous squamous cell carcinoma of the head and neck. Head Neck 2006;28:785-91. [Crossref] [PubMed]
- O'Brien CJ, McNeil EB, McMahon JD, et al. Significance of clinical stage, extent of surgery, and pathologic findings in metastatic cutaneous squamous carcinoma of the parotid gland. Head Neck 2002;24:417-22. [Crossref] [PubMed]
- Thom JJ, Moore EJ, Price DL, et al. The Role of Total Parotidectomy for Metastatic Cutaneous Squamous Cell Carcinoma and Malignant Melanoma. JAMA Otolaryngol Head Neck Surg 2014;140:548-54. [Crossref] [PubMed]
- Franzen A, Buchali A, Lieder A. The rising incidence of parotid metastases: our experience from four decades of parotid gland surgery. Acta Otorhinolaryngol Ital 2017;37:264-9. [Crossref] [PubMed]
- Czerwonka L, De Santis RJ, Horowitz G, et al. Staging cutaneous squamous cell carcinoma metastases to the parotid gland. Laryngoscope 2017;127:2063-9. [Crossref] [PubMed]
- Wang H, Hoda RS, Faquin W, et al. FNA biopsy of secondary nonlymphomatous malignancies in salivary glands: A multi-institutional study of 184 cases. Cancer Cytopathol 2017;125:91-103. [Crossref] [PubMed]
- Franzen A, Lieder A, Guenzel T, et al. The Heterogenicity of Parotid Gland Squamous Cell Carcinoma: A Study of 49 Patients. In Vivo 2019;33:2001-6. [Crossref] [PubMed]
- Chernock RD, Lewis JS. Approach to metastatic carcinoma of unknown primary in the head and neck: squamous cell carcinoma and beyond. Head Neck Pathol 2015;9:6-15. [Crossref] [PubMed]
- McKean ME, Lee K, McGregor IA. The distribution of lymph nodes in and around the parotid gland: an anatomical study. Br J Plast Surg 1985;38:1-5. [Crossref] [PubMed]
- Pisani P, Ramponi A, Pia F. The deep parotid lymph nodes: an anatomical and oncological study. J Laryngol Otol 1996;110:148-50. [Crossref] [PubMed]
- Garatea-Crelgo J, Gay-Escoda C, Bermejo B, et al. Morphological study of the parotid lymph nodes. J Craniomaxillofac Surg 1993;21:207-9. [Crossref] [PubMed]
- Kampel L, Dorman A, Horovitz G, et al. The role of parotidectomy for advanced cutaneous squamous cell carcinoma of the head and neck. Eur Arch Otorhinolaryngol 2021;278:3955-63. [Crossref] [PubMed]
- Kirke DN, Porceddu S, Wallwork BD, et al. Pathologic occult neck disease in patients with metastatic cutaneous squamous cell carcinoma to the parotid. Otolaryngol Head Neck Surg 2011;144:549-51. [Crossref] [PubMed]
- Pollaers K, Davidoss N, Hinton-Bayre A. Management of occult neck disease in metastatic squamous cell carcinoma to the parotid gland. Aust J Otolaryngol 2019;2:24.
- Ebrahimi A, Moncrieff MD, Clark JR, et al. Predicting the pattern of regional metastases from cutaneous squamous cell carcinoma of the head and neck based on location of the primary. Head Neck 2010;32:1288-94. [Crossref] [PubMed]
- Park SW, Eade T, Pang L, et al. Role of neck dissection in metastatic squamous cell carcinoma to the parotid gland. J Laryngol Otol 2016;130:S54-9. [Crossref] [PubMed]
- Hirshoren N, Ruskin O, McDowell LJ, et al. Management of Parotid Metastatic Cutaneous Squamous Cell Carcinoma: Regional Recurrence Rates and Survival. Otolaryngol Head Neck Surg 2018;159:293-9. [Crossref] [PubMed]
- Ch'ng S, Maitra A, Allison RS, et al. Parotid and cervical nodal status predict prognosis for patients with head and neck metastatic cutaneous squamous cell carcinoma. J Surg Oncol 2008;98:101-5. [Crossref] [PubMed]
- Ying YL, Johnson JT, Myers EN. Squamous cell carcinoma of the parotid gland. Head Neck 2006;28:626-32. [Crossref] [PubMed]
- O'Hara J, Ferlito A, Takes RP, et al. Cutaneous squamous cell carcinoma of the head and neck metastasizing to the parotid gland--a review of current recommendations. Head Neck 2011;33:1789-95. [Crossref] [PubMed]
- Melo GM, Guilherme LH, Palumbo MDN, et al. Parotidectomy and neck dissection in locally advanced and relapsed cutaneous squamous cell carcinoma of the head and neck region. Braz J Otorhinolaryngol 2022;88:S152-62. [Crossref] [PubMed]
Cite this article as: Yii RSL, Chai SC, Wan Sulaiman WA, Mat Zain MAB. Cutaneous squamous cell carcinoma with secondary parotid metastasis: a case report. AME Case Rep 2023;7:4.


