
Lymphoepithelial like carcinoma of the anorectal junction, a rare entity associated with human papillomavirus rather than Epstein-Barr virus: a case report
Highlight box
Key findings?
• This case report presents a unique case of LELC in the anal canal which is a rare site for this tumor. High-risk HPV-16 was detected through PCR, whereas EBV was negative on ISH.
What is known and what is new?
• Association of HPV and EBV has been well documented in LELC of the oropharynx and stomach respectively however, there is scarcity of data on association of these viral agents with LELC of the anal canal.
What is the implication, and what should change now?
• LELC is most often not considered in the initial differential diagnosis. This lesion can be mistaken for neuroendocrine carcinoma or medullary carcinoma due to presence of syncytial growth pattern of tumor cells in a lymphoid background. A thorough workup is indicated for lesions with this morphology to reach a correct diagnosis. In addition, more data is needed to establish association of viral agents with LELC of the anal canal.
Introduction
Lymphoepithelial-like carcinomas (LELC) are characterized by undifferentiated large malignant cells with vesicular nuclei and prominent nucleoli, an ill-defined syncytial pattern of growth and prominent non-neoplastic intratumoral lymphoid infiltrate. It is histologically similar to the undifferentiated nasopharyngeal carcinoma but occurs in other anatomic locations such as the lung, salivary gland, breast, urinary bladder, oropharynx, cervix, and mammary gland. In addition, there have been a few reported cases of LELC occurring in the gastrointestinal (GI) tract, with only two cases identified in the anal canal. To our knowledge, this is the third reported case of LELC occurring in the anorectal junction associated with a high-risk human papillomavirus (HPV). We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-53/rc).
Case presentation
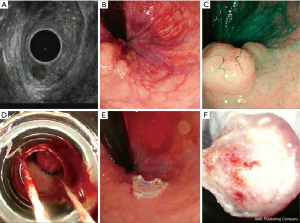
A 58-year-old female with no significant medical history other than hypertension and dyslipidemia presented to the clinic with complaint of rectal pressure for eight months. There was associated mild constipation but no significant history of blood in stool, diarrhea, or weight loss. A pelvic magnetic resonance imaging (MRI) was performed as part of the work-up and documented sigmoid diverticulosis without any other lesions. Endoscopic ultrasound (EUS) with a radial echoendoscope was performed to reveal a single 0.8 cm × 0.7 cm hypoechoic nodule located at the anorectal junction (Figure 1A-1C). Endoscopic mucosal resection (EMR) was performed using Band EMR technique, the polyp was completely retrieved, and specimen was sent for histopathological evaluation (Figure 1D-1F). There was no blood loss, and no target sign was identified during the procedure to indicate colonic perforation.
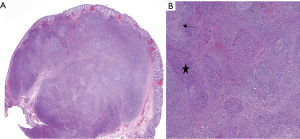
The resected specimen consisted of soft red mucosal tissue measuring 1.3 cm × 1.3 cm × 0.6 cm. The microscopic examination showed a submucosal nodule composed of poorly differentiated cells with large vesicular nuclei, prominent nucleoli, and indistinct cell borders forming a syncytial pattern of growth with associated dense lymphocytic infiltrate and germinal center formation. The overlying squamous and colonic mucosa was unremarkable (Figure 2A,2B).
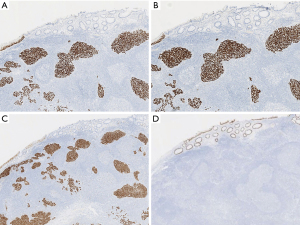
Immunohistochemical analysis revealed diffuse tumor positivity for p16, p40, and p63 (Figure 3A-3C) along with variable co-expression of CK7 and epithelial membrane antigen (EMA). The tumor cells were negative for CDX-2 (Figure 3D), CK20, CEA, chromogranin, synaptophysin, p53, SOX-10, and S100. The Ki-67 proliferation index was relatively high at 50%. In addition, the CD3, CD20, and CD45 adequately highlighted the lymphocytic infiltrate composed of both T- and B-lymphocytes. The neoplastic cells maintained nuclear expression of the mismatch repair (MMR) proteins (MLH1, MSH2, MSH6, and PMS2). Programmed cell death ligand 1 (PD-L1) immunohistochemical staining was performed (with 22C3 clone) and showed expression in 20% of the tumor cells with a combined positive score (CPS) of 40%. Polymerase chain reaction (PCR) for high-risk HPV was positive for HPV-16. In situ hybridization (ISH) for Epstein-Barr virus (EBV)-encoded ribonucleic acid (EBER) was negative.
In summary, the morphologic findings associated with the ancillary testing results represented a rare example of p16/HPV-associated LELC of the anorectal junction. The neoplasm focally involved the inked deep margin of resection.
The patient subsequently underwent extensive radiologic studies to include computed tomography (CT), MRI, and positron emission tomography (PET) scan to reveal no metastatic disease in the body. Of interest, the PET-CT scan showed an area of focal uptake in the anorectal junction, which was interpreted to represent local residual disease. The cancer was staged as T1N0M0. The case was discussed in multidisciplinary tumor board and patient was recommended a course of definitive chemoradiation due to the size and location of tumor and concerns for loss of anal sphincter function with further surgical resection. The chemotherapy regimen included Capecitabine and Mitomycin and received a total radiation dose of 5,040 cGy. Patient developed radiation dermatitis with perianal skin peeling, increased urinary frequency/dysuria, and increased bowel frequency with chemoradiation which resolved with local treatment. Re-staging scans performed 6 weeks post definitive chemoradiation showed no evidence of residual disease and the patient remains asymptomatic 8 months post-diagnosis. The patient will be followed at three to six months interval with anoscopy, inguinal lymph node palpation and digital rectal examination (DRE) for 5 years along with CT scan of the chest, abdomen, and pelvis or MRI annually for three years.
All procedures performed in this study were in accordance with the ethical standards of the institution and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives. However, we followed our Institutional Review Board (IRB) guidelines which exempts case reports with less than three patients from IRB review. All protected Health Information was deidentified as per the Health Insurance Portability and Accountability Act (HIPAA) guidelines in this case report.
Discussion
Lymphoepithelioma, also known as nasopharyngeal carcinoma of the non-keratinizing undifferentiated subtype, is a neoplasm of the nasopharynx characterized by undifferentiated malignant cells with prominent lymphoplasmacytic infiltrate. Hence, neoplasms with similar morphologic features outside the nasopharynx have been regarded as “lymphoepithelial-like” carcinoma (1). LELC has been reported in various locations such as the salivary glands, lung, thymus, oral cavity, vagina, and skin. These lesions have been documented to be associated with viral infection. Specifically, EBV is detected in nasopharyngeal and sinonasal sites, whereas HPV is primarily detected in oropharyngeal and gynecologic areas (2). Prevalence of HPV in LELC vary depending on site. Highest prevalence is noted in LELC of oropharynx where the figures are as high as 86–94% cases being positive for HPV (3). HPV has not been detected in sinonasal LELC.
LELC of the GI tract is relatively rare. A recent review by Zhai et al. identified a total of 2,079 cases of LELC in the US through Surveillance, Epidemiology, and End Results (SEER) data between 1973 to 2015, of which only 39 cases (2%) were reported in the digestive tract (4). The stomach is the most commonly involved region within the GI tract, and more than 80% of the gastric LELC are associated with EBV (1).
LELC in other regions of the GI tract, such as the colon, rectum, and anal canal, are even more exceedingly rare. Up to date, we found 15 reported cases in the literature of which 9 involved the colon, 4 involved the rectum (1,5-16) (Table 1), and only 2 involved the anal canal (17,18) (Table 2). EBV was positive in two and HPV was positive in three of the total cases however, no HPV testing was performed in 10 cases.
Table 1
| S.NO | Author | Age, years | Sex | Site | HPV status | EBV status (ISH) | MSI |
|---|---|---|---|---|---|---|---|
| 1 | Quartino et al. (5) | 45 | F | Rectum | (−) | (−) | NP |
| 2 | Kopparthy et al. (6) | 51 | F | Rectum | (+) | (−) | Intact |
| 3 | Mori et al. (7) | 70 | F | Sigmoid colon | (−) | (−) | NP |
| 4 | Kai et al. (8) | 86 | F | Ascending colon | NP | (−) | NP |
| 5 | Delaney et al. (1) | 85 | F | Sigmoid colon | NP | (−) | Loss of MLH1 and PMS2 |
| 6 | Samaha et al. (9) | 62 | M | Cecum | NP | (−) | NP |
| 7 | De Petris et al. (10) | 44 | M | Right colon | NP | (−) | NP |
| 8 | Palazzo et al. (11) | 29 | M | Rectum | NP | (−) | NP |
| 9 | Kojima et al. (12) | 25 | M | Sigmoid colon | NP | (+) | NP |
| 10 | Vilor et al. (13) | 77 | F | Transverse colon | NP | (−) | NP |
| 11 | Kon et al. (14) | 72 | M | Rectum | NP | (+) | NP |
| 12 | Díaz Del Arco et al. (15) | 86 | F | Splenic flexure | NP | (−) | Loss of MLH1 and PMS2 |
| 13 | Oi et al. (16) | 35 | F | Rectosigmoid | NP | (−) | NP |
S.NO, serial number; HPV, human papillomavirus; EBV, Epstein-Barr virus; ISH, in situ hybridization; MSI, microsatellite instability; F, female; M, male; NP, not performed; (+), positive; (−), negative.
Table 2
| S.NO | Author | Age, years | Sex | Site | HPV status | EBV status (ISH) | MSI |
|---|---|---|---|---|---|---|---|
| 1 | Scott et al. (17) | 68 | M | Anal canal | (+) | (−) | Intact |
| 2 | Weng et al. (18) | 25 | F | Anal canal | (+) | (−) | Intact |
S.NO, serial number; ISH, in situ hybridization; HPV, human papillomavirus; EBV, Epstein-Barr virus; MSI, microsatellite instability; (+), positive; (−), negative; M, male; F, female.
Our current case showed similar findings to the previously reported two cases of LELC of the anal canal (Table 2). In both cases, the patients (aged 68 and 25 years) initially presented with a rectal polyp. The microscopic evaluation revealed a syncytial growth pattern of tumor cells with prominent lymphoid infiltrate. The morphologic features and immunohistochemical staining pattern led to diagnosis of LELC. In addition, both cases were positive for p16 on immunohistochemistry and high-risk HPV-16 on PCR, whereas EBER was negative, and the tumors were microsatellite stable.
Since LELC is such a rare entity in the GI tract, pathologists consider other common primary carcinomas in the initial differential diagnosis, including neuroendocrine carcinoma and poorly differentiated adenocarcinoma. Neuroendocrine tumors are common in the rectum and can present as incidental rectal polyp on endoscopy. They can be distinguished by morphologic features and characteristic immunohistochemical staining with synaptophysin and chromogranin. Poorly differentiated adenocarcinomas are usually not associated with brisk intra-tumoral lymphoid infiltrate and occasionally show a glandular pattern of growth which aid in their identification (18).
The medullary carcinoma of the colon is considered another important differential of LELC, especially given that it typically shows dense lymphoid infiltrate. Medullary carcinomas resemble neuroendocrine carcinoma with sheets, cords, or organoid growth patterns; however, they are negative for the neuroendocrine markers. The tumor cells have abundant eosinophilic cytoplasm with vesicular nuclei and prominent nucleoli. Due to prominent lymphoid infiltrate and negativity for CDX2, LELC can be easily mistaken for medullary carcinoma. However, medullary carcinoma tends to have sheets of tumor cells with well-defined margins, and the lymphoid infiltrate is predominantly peri-tumoral, whereas LELC makes small clusters of tumor cells with prominent intra-tumoral lymphoid infiltrate (19). Immunohistochemistry is also helpful in differentiating these two entities as medullary carcinoma is negative for CK5/6, p40, and p63 which are markers of squamous differentiation and are positive in LELC. Medullary carcinoma also has high microsatellite instability with loss of staining for MLH1 (8).
Since LELC are rare in the anal canal, no management guidelines or standard treatments have been established. Endoscopic resection and concurrent radiotherapy are the modalities of treatment described in most case reports on LELC (18). The two patients from the published case reports of LELC of the anal canal were also treated with chemoradiation. One patient was treated with local resection and chemoradiation and patient remained asymptomatic with no evidence of metastasis or recurrence eight months post-surgical resection while no follow up data was available for the other patient. Our patient was treated with chemoradiation similar to what has been recommended for squamous cell carcinomas.
Conclusions
Undifferentiated carcinoma with prominent lymphoid stroma in the anal canal should raise a concern for LELC. However, due to its rarity in the anal canal, further studies are required to establish an association with viral etiologic agents like EBV or HPV.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-53/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-53/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institution and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives. However, we followed our Institutional Review Board (IRB) guidelines which exempts case reports with less than three patients from IRB review. All protected Health Information was deidentified as per the HIPAA guidelines in this case report.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Delaney D, Chetty R. Lymphoepithelioma-like carcinoma of the colon. Int J Clin Exp Pathol 2012;5:105-9.
- Rytkönen AE, Hirvikoski PP, Salo TA. Lymphoepithelial carcinoma: two case reports and a systematic review of oral and sinonasal cases. Head Neck Pathol 2011;5:327-34. [Crossref] [PubMed]
- Acuña G, Gomà M, Temprana-Salvador J, et al. Human papillomavirus in laryngeal and hypopharyngeal lymphoepithelial carcinoma. Mod Pathol 2019;32:621-6. [Crossref] [PubMed]
- Zhai X, Liu J, Lu D, et al. Demographics, clinical features, and prognosis of rare lymphoepithelioma-like carcinoma across different anatomic sites. J Egypt Natl Canc Inst 2022;34:5. [Crossref] [PubMed]
- Quatrino G, Kampagianni O, Boudreaux CW. Lymphoepithelial-Like Carcinoma Involving a Rectal Tonsil. Clin Oncol 2016;1:3.
- Kopparthy P, Chaffin J, Feely MM, et al. HPV-16-associated lymphoepithelial-like carcinoma mimicking rectal tonsil. Clin J Gastroenterol 2021;14:810-4. [Crossref] [PubMed]
- Mori Y, Akagi K, Yano M, et al. Lymphoepithelioma-like carcinoma of the colon. Case Rep Gastroenterol 2013;7:127-33. [Crossref] [PubMed]
- Kai K, Hidaka H, Nakamura T, et al. A case of poorly differentiated adenocarcinoma with lymphoid stroma originated in the ascending colon diagnosed as lymphoepithelioma-like carcinoma. Clin J Gastroenterol 2020;13:538-44. [Crossref] [PubMed]
- Samaha S, Tawfik O, Horvat R, et al. Lymphoepithelioma-like carcinoma of the colon. Dis Colon Rectum 1998;41:925-8. [Crossref] [PubMed]
- De Petris G, Lev R, Quirk DM, et al. Lymphoepithelioma-like carcinoma of the colon in a patient with hereditary nonpolyposis colorectal cancer. Arch Pathol Lab Med 1999;123:720-4. [Crossref] [PubMed]
- Palazzo JP, Mittal KR. Lymphoepithelioma-like carcinoma of the rectum in a patient with ulcerative colitis. Am J Gastroenterol 1996;91:398-9.
- Kojima Y, Mogaki M, Takagawa R, et al. A case of lymphoepithelioma-like carcinoma of the colon with ulcerative colitis. J Gastroenterol 2007;42:181-5. [Crossref] [PubMed]
- Vilor M, Tsutsumi Y. Iocalization of Epstein-Barr virus genome in lymphoid cells in poorly differentiated adenocarcinoma with lymphoid strorna of the colon. Pathol Int 1995;45:695-7. [Crossref] [PubMed]
- Kon S, Kasai K, Tsuzuki N, et al. Lymphoepithelioma-like carcinoma of rectum: possible relation with EBV. Pathol Res Pract 2001;197:577-82. [Crossref] [PubMed]
- Díaz Del Arco C, Esteban Collazo F, Fernández Aceñero MJ. Lymphoepithelioma-like carcinoma of the large intestine: A case report and literature review. Rev Esp Patol 2018;51:18-22. [Crossref] [PubMed]
- Oi H, Yamamoto S, Kono Y, et al. A case of Lymphoepithelioma-like carcinoma (LELC) developed in the rectum. Rep Pract Oncol Radiother 2019;24:624-8. [Crossref] [PubMed]
- Scott K, Trainor J, McVeigh G, et al. Human Papillomavirus (HPV)-associated Lymphoepithelioma-like Carcinoma of the Vagina and Anal Canal: A Rare Variant of Squamous Cell Carcinoma. Int J Gynecol Pathol 2019;38:183-8. [Crossref] [PubMed]
- Weng W, Sheng W, Wang L. Human Papillomavirus-Associated Lymphoepithelioma-Like Carcinoma of the Anal Canal: A Case Report and Literature Review. Front Med (Lausanne) 2021;8:766960. [Crossref] [PubMed]
- Chetty R. Gastrointestinal cancers accompanied by a dense lymphoid component: an overview with special reference to gastric and colonic medullary and lymphoepithelioma-like carcinomas. J Clin Pathol 2012;65:1062-5. [Crossref] [PubMed]
Cite this article as: Ali SM, Park BU, Kallakury B. Lymphoepithelial like carcinoma of the anorectal junction, a rare entity associated with human papillomavirus rather than Epstein-Barr virus: a case report. AME Case Rep 2023;7:3.


