
Case report: multiple lesions during navigation bronchoscopy; seen one, seen them all?
Highlight box
Key findings
• Performing inspection bronchoscopy preceding to navigation bronchoscopy for a suspicious peripheral lesion can give important added information.
• An endobronchial approach allows targeting of multiple bilateral peripheral lesions in one session while also allowing evaluation of lymph nodes.
• A patient with three separate primary tumors was treated with three different treatment modalities.
What is known and what is new?
• No structured guidelines exist for the diagnoses of multiple nodules.
• This case report suggests that a more structured guideline for the workup of multiple nodules is warranted.
What is the implication, and what should change now?
• Navigation bronchoscopy can play an important role in the diagnoses of multiple intrapulmonary nodules.
• Possible future bronchoscopic mediated local therapies may offer further patient tailored approaches to diagnoses and treatment of multiple pulmonary nodules.
Introduction
The diagnosis of peripheral pulmonary nodules remains a challenging procedure. These nodules are often detected as multiple synchronous nodules in a single patient, with a recent large prospective navigation bronchoscopy trial by Folch et al. showing that in 13% of cases multiple lesions needed to be sampled (1). Before the development of reliable image guided transbronchial biopsy, the only available procedure with high yield was computed tomography (CT) guided transthoracic biopsy (TTNB). TTNB is however difficult in small lesions and has a high complication rate that increases with the number of passes through the pleura and is therefore seldom performed for multiple lesions and never for bilateral nodules in one session (2).
Cone-beam CT guided navigation bronchoscopy (CBCT-NB) in the hybrid operating theatre may offer a solution in cases where multiple lesions are seen. CBCT-NB is a navigation bronchoscopy technique in which the precise imaging capabilities of CBCT are combined with (augmented) fluoroscopy for both navigating instruments near to the lesion followed by the ability to precisely confirm positioning of consecutive sampling tools through CBCT. These characteristics result in a high diagnostic yield for patients with peripheral pulmonary nodules. The endobronchial approach reduces the risk of pneumothorax significantly, with only a 1.5% risk of pneumothorax for navigation bronchoscopy being reported (3). This combination of test characteristics makes CBCT-NB well suited to sample multiple lesion in one session, as shown by Verhoeven et al. where 22% of all patients had multiple lesions sampled in one session (4). CBCT-NB can furthermore be combined with separate procedures such as an inspection bronchoscopy or a linear endobronchial ultrasound examination (EBUS) of the mediastinal and hilar lymph nodes, creating the possibility of a comprehensive examination including both diagnosis and staging for suspected early-stage lung cancer. In this case report, we will first describe our routine workflow of CBCT-NB followed by a case description presenting a unique case with three different synchronous primary tumors treated with three different treatment modalities that highlights the added value of CBCT-NB in the diagnostic work-up of suspected early-stage lung cancer. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-40/rc).
Workflow
Our normal workflow starts before the procedure in the hybrid operating theatre. The first step is to analyze the preoperative CT scan and memorize the optimal route towards the target nodule, even in cases where adjunct technology like electromagnetic navigation technology is used. This helps initial coarse navigation and decreases procedure time.
All procedures are performed under general anesthesia, preferably using a laryngeal mask (for consecutive EBUS-TBNA assessment). If needed, intubation can also be performed. Ventilation is optimized to minimize atelectasis, with high positive end-expiratory pressure (PEEP), bigger volumes with low breathing frequency and a low FiO2%, based on ventilation recommendations as described by Pritchett et al. (5).
After topical lidocaine application, a routine inspection bronchoscopy is performed to check for endobronchial abnormalities and possible occult disease. After inspection the bronchoscope is moved towards the subsegment where the target nodule is located. An extended working catheter (EWC) with a passive angulated tip is passed through the working channel and inserted in the target airway. Based on the pre-procedural plan, initial coarse navigation through multiple bifurcations of the airway in the direction of the nodule is performed based on pre-procedural memorization and coarse fluoroscopy. The first CBCT scan is subsequently performed, generally with the intention to obtain high quality imaging to ensure optimal visibility of the peripheral airways and the nodule. After the CBCT spin, the target nodule and the airway leading towards the nodule are segmented, which is subsequently augmented on fluoroscopy as an overlay. Further navigation is performed using the augmented fluoroscopy in multiple directions as a guidance. When navigation is considered finalized, radial endobronchial ultrasound (rEBUS) is utilized as an additional tool to confirm tumor access. When rEBUS is unable to visualize the tumor, or when there is doubt about the position of the EWC, a CBCT scan can repeatedly be acquired to check the position in regard to the lesion. These repeated scans are generally collimated, meaning only the minimally required area needed is scanned with as low a dose as possible following the ALARA principles (as low as reasonably achievable). Sampling is subsequently performed with tools at the discretion of the operator, with needle, forceps biopsy, brush and cryobiopsy as available options. Rapid onsite evaluation (ROSE) is utilized to determine if representative samples are taken. In general, more than ten samples are taken as we have previously shown that the number of samples significantly influences diagnostic yield (6). After samples have been taken, navigation to a second nodule can be indicated. The initial CBCT scan is then routinely used to segment the new target along with its associated endobronchial pathway. If trans-parenchymal navigation is believed necessary, the pathway is segmented until that point after which an update collimated CBCT will then be made.
Case presentation
This 60-year-old asymptomatic male patient presented in our outpatient department because of a suspicion of primary lung cancer as discovered on a chest X-ray performed by his general practitioner. He was an active smoker and had signs of an old myocardial infarction on electrocardiography (EKG) but no other relevant medical history. His physical examination did not show any abnormalities.
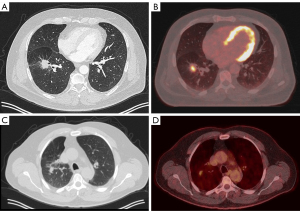
The screening X-ray showed a shadow in the right upper lobe (RUL) for which the patient was referred to a pulmonologist. A subsequent CT-thorax showed two nodules: one in the right lower lobe with a long axis diameter of 30 mm and one in the RUL with a long axis diameter of 17 mm. Both nodules were deemed of sufficient high risk of lung cancer to warrant further analysis. A positron emission tomography (PET)-scan was therefore performed which showed both identified nodules to be fluorodeoxyglucose (FDG)-avid, without mediastinal involvement or other signs of metastatic disease (see Figure 1).
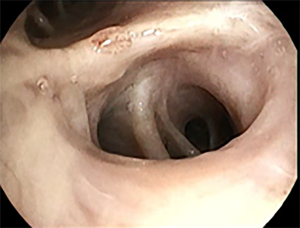
Patient was subsequently referred for minimal invasive biopsy of these nodules. A navigation bronchoscopy was considered most optimal, because both nodules could then be sampled in one procedure. After induction of general anesthesia, an inspection bronchoscopy was performed following the workflow as described above. While it was expected that only the right side was affected, inspection bronchoscopy revealed a third adverse finding. During the routine inspection, a submucosal lesion with dotted vessels of approximately 5 mm was observed in the left upper lobe (LUL). Based on its appearance, sampling for further analysis was believed indicated (7,8). Repeated sampling using a forceps biopsy instrument was performed (see Figure 2).
Subsequently, the navigation bronchoscopy procedure ensued. An EWC (Medtronic) with a 180 degrees angulated distal tip was inserted in the target segment of the RUL and a first CBCT spin was performed. The target nodule and route were segmented for further image guided navigation which in this case required several CBCT scans for repositioning of the catheter in order to access this RUL nodule. Lesion access was confirmed with rEBUS and CBCT (Figure 3). Subsequently, this lesion was sampled with transbronchial needle aspiration and (new) forceps biopsy providing both cytological and histological samples. As ROSE was not able to provide a certain diagnosis, the lesion was sampled extensively for histology.
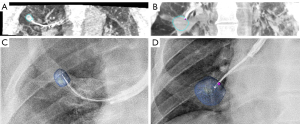
Next, for the second nodule, the EWC was inserted in the target segment of the right lower lobe (RLL) using augmented fluoroscopy for the second nodule and its planned route which were segmented on the initial high dose CBCT. Navigation towards this lesion resulted in immediate access of the nodule and position confirmation was obtained by rEBUS and a new CBCT spin (Figure 3). Both a new TBNA needle and biopsy forceps were used to prevent cross-contamination for sampling of this third target. ROSE did provide a preliminary diagnosis suspicious for malignancy in the second nodule. At the end of the procedure, an X-ray was obtained using the CBCT system to check for signs of a pneumothorax, which were not found. Although there was no imaging based suspicion of lymphadenopathy, a systematic linear EBUS examination of the mediastinal and hilar lymph nodes was performed to check for signs of nodal disease. All lymph node regions showed small lymph nodes (<5 mm) with benign B-mode characteristics, which is why no fine needle aspiration was performed. After observation in day care setting, the patient was discharged the same day without complications.
Pathological analysis of tissue samples revealed the endobronchial lesion in the LUL as a squamous cell carcinoma of the lung based on immunohistochemistry, tropomyosin receptor kinase (TRK) positive, tumor protein 53 (TP53) positive, programmed death ligand-1 (PD-L1) <1%. The RUL nodule was diagnosed as a non-small cell carcinoma of the lung not otherwise specified (NOS), with no further differentiation possible due to a low malignant cell count which precluded further molecular and clonal analysis. The lesion in the RLL was diagnosed as an adenocarcinoma of the lung, thyroid transcription factor 1 (TTF1) positive with PD-L1 <1%. There was insufficient material to perform clonal analysis on the two parenchymal lesions. Based on the pathology findings, and the radiological and clinical presentation of the lesion, the multidisciplinary tumor board including the pathologist concluded that it was most likely that this patient suffered from three separate primary pulmonary malignancies. They were respectively staged as: RUL cT1bN0M0, RLL cT1cN0M0 and endobronchial: cT1aN0M0. Although pathological examination was not able to fully differentiate if the non-small cell lung cancer (NSCLC) NOS of the RUL was a third primary or an ipsilateral metastasis of the RLL lesion, it was decided to not perform repeat biopsy of the RUL nodule to prevent further diagnostic delay.
Due to the uncommon presentation, a cerebral magnetic resonance imaging (MRI) was performed which ruled out cerebral metastases. Subsequently, an additional mediastinoscopy showed no lymph node metastases.
Because the patient did not have the pulmonary reserve to safely undergo a right sided pneumonectomy, a video assisted thoracoscopic surgical lobectomy was performed to resect the RLL. Stereotactic ablative radiotherapy was used to treat the lesion in the RUL, and the endoluminal squamous cell carcinoma was treated with endobronchial cryoablation. Unfortunately, the lymph node dissection performed during surgery of the RLL lesion revealed nodal metastases in levels 10 right and 11 right resulting in a final staging of pT1cN1 PL0 R0. Due to this upstaging, the patient received adjuvant chemotherapy in the form of carboplatin and pemetrexed for four cycles. No severe adverse events took place during the diagnostic process and following treatment. Patient is currently more than 12 months post-treatment and has no signs of recurrence, both on imaging and repeated bronchoscopy and re-biopsy of the endoluminally treated squamous cell carcinoma. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
This case study shows that navigation bronchoscopy is a diagnostic procedure that allows for a patient tailored approach to diagnose (and potentially treat) early-stage lung cancer. Although rare, this patient was eventually diagnosed with three separate primary malignancies and was treated with curative intent using three different treatment modalities. This resulted in the least amount of treatment related morbidity, and the patient has been disease free with more than 12 months of follow-up.
This case highlights some of the benefits which navigation bronchoscopy offers over the more widely implemented CT guided TTNB. Firstly, a strength is that multiple nodules could be sampled in one session. Additionally, navigation bronchoscopy can be combined with other procedures to optimize the diagnostic process. in this case report, a routine endobronchial inspection discovered occult disease, which would otherwise most likely have only been diagnosed in an advanced stage of disease. A limitation is that there is no standardized workup for multiple nodules. Guidelines such as the British thoracic society (BTS) give recommendations on the imaging and follow-up of multiple nodules, but specific guidelines for diagnosis and treatment of multiple nodules are lacking (9). Furthermore, there is little information on how often multiple different primary cancers truly occur. Guidelines have proposed algorithms to differentiate between second primaries and intrapulmonary metastasis (10), but there is little information on the incidence of multiple primaries. This makes an estimation on how often CBCT-NB could be utilized effectively for similar cases difficult.
Thinking of future perspectives, endobronchial treatment can also be included to further optimize a patient tailored approach (11). If preprocedural evaluation of the patient already showed that both nodules cannot be resected, bronchoscopic mediated local therapies may be used as a future alternative, especially in cases where radiotherapy is not a good alternative.
Conclusions
In conclusion, CBCT-NB offers a safe, and minimal invasive diagnostic technique with the potential to diagnose multiple different lesions in one session, reducing treatment delay and optimizing patient tailored treatment.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Calvin Ng and Joyce Chan) for the series “Case Reports in Hybrid Operating Theatre” published in AME Case Reports. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-40/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-40/coif). The series “Case Reports in Hybrid Operating Theatre” was commissioned by the editorial office without any funding or sponsorship. No personal fees have been received by EFMvdH, SEPK or RLJV. All subsequent described fees and funding have been received by the research institution where researchers are employed. RLJV and EFMvdH have received funding for research by Philips, AstraZeneca, Johnson & Johnson, Pentax, Galvanize Therapeutics and Bioncise. SEPK, EFMvdH and RLJV have received consultancy fees by Johnson & Johnson. EFMvdH has received consultancy fees by Philips. EFMvdH has received support for travel and fees for lectures by Pentax and fees for lecture by Janssen Cilag. EFMvdH is an unpaid board member of the WABIP and EABIP. RLJV has received fees for lecture by Medtronic and support for travel by Pentax. RLJV is an unpaid board member of the Dutch society of technical physicians. RLJV and EFMvdH have patents pending. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Folch EE, Bowling MR, Pritchett MA, et al. NAVIGATE 24-Month Results: Electromagnetic Navigation Bronchoscopy for Pulmonary Lesions at 37 Centers in Europe and the United States. J Thorac Oncol 2022;17:519-31. [Crossref] [PubMed]
- Heerink WJ, de Bock GH, de Jonge GJ, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 2017;27:138-48. [Crossref] [PubMed]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [Crossref] [PubMed]
- Verhoeven RLJ, Fütterer JJ, Hoefsloot W, et al. Cone-Beam CT Image Guidance With and Without Electromagnetic Navigation Bronchoscopy for Biopsy of Peripheral Pulmonary Lesions. J Bronchology Interv Pulmonol 2021;28:60-9. [Crossref] [PubMed]
- Pritchett MA, Lau K, Skibo S, et al. Anesthesia considerations to reduce motion and atelectasis during advanced guided bronchoscopy. BMC Pulm Med 2021;21:240. [Crossref] [PubMed]
- Verhoeven RLJ, van der Sterren W, Kong W, et al. Cone-beam CT and Augmented Fluoroscopy-guided Navigation Bronchoscopy: Radiation Exposure and Diagnostic Accuracy Learning Curves. J Bronchology Interv Pulmonol 2021;28:262-71. [Crossref] [PubMed]
- van der Heijden EHFM, Candoli P, Vasilev I, et al. Image enhancement technology in bronchoscopy: a prospective multicentre study in lung cancer. BMJ Open Respir Res 2018;5:e000295. [Crossref] [PubMed]
- van der Heijden EH, Hoefsloot W, van Hees HW, et al. High definition bronchoscopy: a randomized exploratory study of diagnostic value compared to standard white light bronchoscopy and autofluorescence bronchoscopy. Respir Res 2015;16:33. [Crossref] [PubMed]
- Callister ME, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70:ii1-54. [Crossref] [PubMed]
- Detterbeck FC, Franklin WA, Nicholson AG, et al. The IASLC Lung Cancer Staging Project: Background Data and Proposed Criteria to Distinguish Separate Primary Lung Cancers from Metastatic Foci in Patients with Two Lung Tumors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:651-65.
- Chan JWY, Lau RWH, Ngai JCL, et al. Transbronchial microwave ablation of lung nodules with electromagnetic navigation bronchoscopy guidance-a novel technique and initial experience with 30 cases. Transl Lung Cancer Res 2021;10:1608-22. [Crossref] [PubMed]
Cite this article as: Kops SEP, Verhoeven RLJ, van der Heijden EFM. Case report: multiple lesions during navigation bronchoscopy; seen one, seen them all? AME Case Rep 2023;7:2.


