Effect of oral enzyme combination, diet and exercise on chronic low-grade inflammatory conditions—a report of three cases
Introduction
The awareness of systemic chronic inflammation (SCI) has increased, as recent studies show that SCI can lead to widespread disorders such as type 2 diabetes, cardiovascular disease and chronic kidney disease, among others (1-3). These prevalent diseases are a major cause of morbidity and mortality worldwide (1) and thus impose a great financial burden on health care systems (4-7). Inflammation is the body’s main defense mechanisms against external agents such as bacteria, viruses, toxins and infections. Usually, the inflammatory state resolves after the trigger has been eliminated (8). However, some diseases prevent the resolution of inflammation which leads to a state of low-grade inflammation that can persist for a long time (1). The physiological purpose of inflammation, which is a defense against harmful stimuli, then turns into a pathological state that ultimately causes tissue damage. The emergence of chronic low-grade inflammation is mostly enabled by the current life conditions of the patient such as social, psychological, environmental as well as biological factors and therefore holds a great potential for prevention (1,9). It has been described that dietary factors can have a great impact on inflammation and that changing harmful nutritional habits in combination with exercising to reduce fatty tissue can prevent inflammatory conditions (10). Although a consensus as to which markers best represent low-grade inflammation has not been reached yet (10), SCI may be associated with an elevation of inflammatory cytokines in the serum (11).
In order to start the effective treatment of SCI conditions, it would be enough to apply some changes to the lifestyle. The main ones consist of changes in the dietary pattern: diet rich in vitamins, minerals, fiber, antioxidants, folates, polyphenols and omega-3 fatty acids and low in trans fatty acids, simple sugars, animal proteins and processed foods correlates inversely with pro-inflammatory cytokine levels; furthermore, an overall reduction in caloric intake, the constant performance of moderate aerobic physical activity, individually adapted based on the patient’s preferred and manageable type of activity, and better stress management should be added.
Also, oral enzyme combination (OEC) has been proven to be efficacious in treating pain and acute inflammation associated with inflammatory conditions of the musculoskeletal system (12-14). Bromelain, a cysteine protease derived from pineapples, and trypsin, a serin protease isolated from porcine pancreas, are enzymes with a broad spectrum of application. A common addition is the flavonoid rutin, a powerful antioxidant and anti-inflammatory plant secondary metabolite present in e.g., Sophora japonica, the Japanese pagoda tree (15). Proposed mechanisms of action include activation of the ubiquitous antiprotease alpha-2-macroglobulin, enabling binding of cytokines and controlling signaling pathways (16,17), and direct proteolytic effects on surface receptors or advanced glycation end products (AGEs) (18).
Remarkably, OECs have been clinically shown to reduce pain in knee osteoarthrosis comparable to diclofenac (19). A meta-analysis of six trials also confirmed this, showing that while as effective as diclofenac, OEC had a favorable tolerability and safety profile with a significantly lower risk of treatment-emergent adverse events, related study discontinuations, and changes in laboratory parameters (12). Systemic anti-inflammatory effects of OEC were also confirmed in another study, where it significantly reduced serum IL-6 levels, compared to placebo (20).
Overall, OEC has been shown to reduce edema, exudate and pain, common symptoms of inflammation, and are able to actively support and accelerate the controlled physiological progressions to restore the physiological balance (18,21).
In the presented case report, we applied a combination of diet, exercising and, as food supplement, an OEC of bromelain, trypsin and the flavonoid rutoside1 to treat SCI, reduce body weight as well as maximize the body’s own regenerative healing mechanisms and therapeutic effect. Three patients with inflammatory conditions and polyarthralgia were treated with this combinatory approach, additionally to recommended and scientifically validated standard treatment. C-reactive protein (CRP) is a well-established and easily measurable biomarker for different inflammatory conditions such as obesity, type 2 diabetes, rheumatoid arthritis, psoriasis as well as Crohn’s disease (10) and was therefore used in our report to quantify inflammation. We present the following cases in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-45/rc) (22).
Case presentation
The cases described have been part of a routine outpatient activity. Their description could be of interest in the planning of future studies on this topic. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the three patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Case 1: persisting asthenia and polyarthralgia
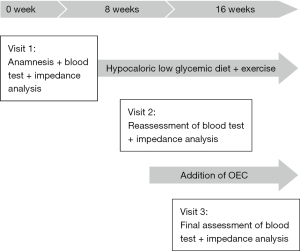
A 65-year-old treatment-naive male sought medical treatment because of asthenia and abdominal swelling. He presented with overweight (body mass index, BMI: 29.22) and polyarthralgia of the large joints (shoulders, hips and knees). Blood test detected prediabetes (HbA1c: 6.5%) as well as high CRP (5.5 mg/dL). Bioelectrical impedance analysis was performed using an Akern® BIA measuring device (Pontassieve, Italy) to decide on the first line treatment. Therapeutic intervention consisted of a hypocaloric low glycemic diet and exercise therapy with moderate aerobic activity, i.e., cycling for 45 minutes a day for 8 weeks. At visit 2, bioelectrical impedance analysis and blood tests were repeated to assess HbA1c and CRP. Only CRP remained above tolerance range (Table 1), while HbA1c fell within normal range limits.
Table 1
| Visit 1, anamnesis | Visit 2, diet + exercise therapy | Visit 3, diet + exercise therapy + OEC | |
|---|---|---|---|
| Weight (kg) | 89.5 | 87 | 85.3 |
| BMI, kg/m2 | 29.22 | 28.41 | 27.85 |
| FM (%) | 36.5 | 35.00 | 33.00 |
| CRP (mg/dL) | 5.5 | 5.3 | 1.2 |
| HbA1c (%) | 6.5 | 5.8 | 5.5 |
OEC, oral enzyme combination; BMI, body mass index; CRP, C-reactive protein; FM, fat mass; HbA1c, glycosylated hemoglobin.
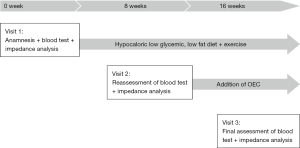
As the asthenia, polyarthralgia and inflammation persisted, the initial treatment was supplemented with OEC (2 tablets twice a day) for a further 8 weeks period. According to his own statement, the patient complied firmly to the therapy. At visit 3, CRP was reduced distinctly and fell within normal limits (Table 1). The patient reported a clear improvement of asthenia and the complete disappearance of the polyarthralgia. No adverse events occurred. The course of patient care and treatment is displayed in Figure 1.
Case 2: psoriatic lesions and polyarthralgia
A 51-year-old male with psoriatic skin lesions (Figure 2) as well as polyarthralgia of the small joints of the hands characterized by swelling and pain presented to our clinic because he was not satisfied with treatment outcome so far for psoriatic arthritis and because of the need for assistance with dieting. A blood test detected elevated CRP (6.5 mg/dL), which indicated a low-grade inflammation. Bioelectrical impedance analysis was performed to decide on the first line treatment. The first treatment approach consisted in a gluten-free diet, since gluten sensitivity was suspected. The diet was also normocaloric with a low glycemic index as well as with limited intake of dairy, as lactose intolerance was also suspected, and abstinence from alcohol. Skin lesions showed a clear improvement just after the onset of dietary treatment. Exercise therapy with moderate aerobic activity (4 times a week for 45 minutes) was initiated at visit 2 for a period of 8 weeks. Since the polyarthralgia was still present at visit 2, the initial treatment was supplemented with OEC (2 tablets twice a day) for an additional 8 weeks. Each treatment period was followed by a reassessment of the blood tests and bioelectrical impedance analysis. Additionally, the joint pain was evaluated with a visual analogue scale (VAS, 0: no pain; 10: worst pain). At visit 3, the patient reported a clear improvement of polyarthralgia symptoms. Blood tests showed that CRP was reduced to normal levels only after 8 weeks of diet/exercise therapy in combination with enzyme therapy (Table 2). The psoriatic lesions have gone into complete remission (Figure 3).
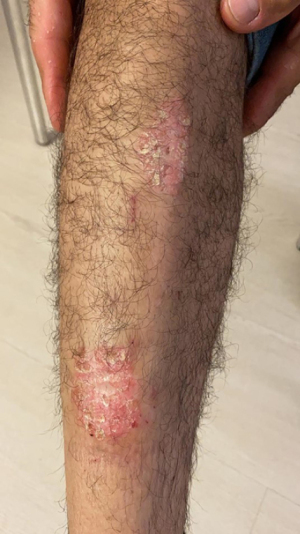
Table 2
| Parameters | Visit 1, anamnesis | Visit 2, diet + exercise therapy | Visit 3, diet + exercise therapy + OEC | Visit 4, OEC | Visit 5, OEC |
|---|---|---|---|---|---|
| Weight (kg) | 63.5 | 62.8 | 63.2 | 64.1 | 63.5 |
| BMI, kg/m2 | 22.2 | 22 | 22.1 | 22.3 | 22.2 |
| FM (%) | 18.5 | 17.00 | 19.00 | 18 | 18.5 |
| CRP (mg/dL) | 6.5 | 6.3 | 2.1 | 4.5 | 1.5 |
| VAS | 6 | 7 | 1 | 5 | 3 |
OEC, oral enzyme combination; BMI, body mass index; CRP, C-reactive protein; FM, fat mass; VAS, visual analogue scale.
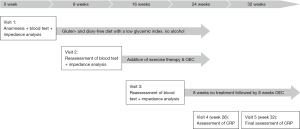
OEC therapy was then interrupted for the subsequent eight weeks since inflammation seemed to have resolved. However, on reassessment, the patient reported the reappearance of the joint pain a few days after interrupting OEC therapy. OEC was therefore resumed for another 8 weeks. Only 2 weeks after resumption of OEC treatment, CRP was 4.5 at visit 4. After another 6 weeks, CRP declined to 1.5 (Table 2). The course of patient care and treatment is displayed in Figure 4.
Case 3: knee osteoarthritis (OA) and obesity
A 62-year-old obese male with moderate to severe knee OA sought medical treatment with the aim of reducing body weight as previously advised by his orthopedic surgeon. At visit 1, the patient’s body weight was 97 kg (fat mass: 38%) and blood tests showed CRP of 7.8 mg/dL as well as HbA1c of 7.2% (Table 3), which indicated diabetes. Erythrocyte sedimentation rate, fibrinogen and leucocyte count were each in a normal range (data not shown). The patient reported long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) for the management of joint pain with an average use of 3–4 tablets of Ibuprofen 600 mg a week. After an 8-week period, during which the patient was recommended a hypocaloric diet with a low glycemic index, low content of saturated fatty acids and an increased intake of fiber and exercise therapy, CRP was found to still be 7.6 mg/dL, while the HbA1c was 7%. The patient’s weight remained stable (96.5 kg) as well as his fat mass (38.3%), as his compliance to the diet was rather poor. In addition, pain in the knee remained substantially unchanged, as well as the weekly dosage of NSAIDs.
Table 3
| Visit 1, anamnesis | Visit 2, diet + exercise therapy | Visit 3, diet + exercise therapy + OEC | |
|---|---|---|---|
| Weight (kg) | 97 | 96.5 | 97.2 |
| BMI, kg/m2 | 32.8 | 32.6 | 32.9 |
| FM (%) | 38 | 38.3 | 38.5 |
| CRP (mg/dL) | 7.8 | 7.6 | 4.2 |
| HbA1c (%) | 7.2 | 7 | 6.4 |
| NSAID use | 1,800 to 2,400 mg/week | 1,800 to 2,400 mg/week | 600 mg/week |
OEC, oral enzyme combination; BMI, body mass index; FM, fat mass; CRP, C-reactive protein; HbA1c, glycosylated hemoglobin; NSAID, non-steroidal anti-inflammatory drugs.
The initial treatment was supplemented with OEC (2 tablets twice a day) for a further 8 weeks period. The reassessment of blood parameters showed a decrease of the ongoing inflammation as CRP was reduced to 4.2 mg/dL. A slight reduction in HbA1c was also detected, switching from diabetes to prediabetes range. Patient’s weight as well as fat mass did not improve as compliance with the diet was still assessed as poor. Conversely, compliance to OEC treatment was optimal. The patient reported a clear improvement in the knee pain, with a reduced frequency of NSAID intake (Table 3) as well as no adverse events. The course of patient care and treatment is displayed in Figure 5.
Discussion
Systemic chronic low-grade inflammation is a common pathological condition increasingly affecting patients around the world. It is related to obesity, type 2 diabetes, atherosclerosis, malignant neoplasms, and neurodegenerative diseases (1-3). The inflammatory response may be manifested as pain, swelling, redness and heat (23). The three patients presented in this case report sought medical treatment because of asthenia and polyarthralgia as well as arthritis, psoriasis and obesity. All these conditions and their associated symptoms are accompanied by a low-grade inflammatory status which leads to a restriction of health-related quality of life in the long term (24). Our approach was to first quantify the inflammatory status using CRP as a biomarker. It is well established that CRP can be used as a biomarker for acute infection, inflammatory processes as well as for cardiovascular events (25). The role of CRP in inflammatory diseases has been described in several studies.
CRP has for example been shown to increase up to 1,000-fold in infectious and inflammatory conditions (26). In a systematic review and meta-analysis, CRP levels in patients with OA were significantly associated with pain and decreased physical function (27). The authors state that low-grade inflammation characterized by elevated CRP levels plays a crucial role in the experience of symptoms in OA (27). In our case report, all three patients reported frequent pain. Treatment with OEC was able to reduce NSAID intake from up to 2,400 to 600 mg/week in our patient with knee OA (case 3) as well as to reduce CRP strongly in all three patients, indicating a highly effective pain relief and decrease in inflammation. A cohort study on OA with 1,335 participants older than 55 years showed that higher levels of CRP increased the risk for incidence and progression of OA significantly (28).
It has also been described that CRP levels, among others, are significantly correlated to BMI and age (28). A BMI >30 kg/m2 is defined as obesity (29). Its prevalence has been rising worldwide since the last decades with an increasingly earlier onset (29). Several studies confirm that there is a link between increased CRP levels and high body weight since adipocytes produce tumor necrosis factor α and interleukin 6, which stimulate CRP release (30). Over time, when adipose tissue, especially visceral adipose tissue, expands, more immune cells infiltrate into the tissue leading to local and systemic low-grade inflammation (30). In our case report, high CRP levels indeed correlated with obesity, as two obese patients exhibited elevated CRP values.
Low-grade inflammation characterized by high CRP is also associated with the pathogenesis of type 2 diabetes (31,32). Our patient from case 1 with apparent prediabetes benefitted from the prescribed diet and exercise program concerning his body weight and HbA1c. However, only addition of OEC was effective in treating the predominant low-grade inflammation and by CRP. Our patient from case 3 with apparent diabetes benefitted greatly from OEC since CRP and HbA1c were reduced clearly after addition of OEC. Compliance to OEC was good while compliance to diet and exercise was rather poor.
Psoriasis is an inflammatory disease whose central feature is the lack of inflammation-resolving mechanisms with eicosanoid mediators involved (29). Proinflammatory eicosanoids are formed from arachidonic acid (AA; omega-6), while eicosapentaenoic acid (EPA; omega-3) leads to the formation of anti-inflammatory eicosanoids. The AA/EPA balance thus plays an essential immunological role (29,33). Also, increased intake of simple sugars, high levels of fat and alcohol consumption can exacerbate psoriatic lesions or psoriasis-associated symptoms like anxiety (34). A nutritional therapeutical approach is therefore indicated in psoriasis (29) with a personalized diet for every patient (34). Our initial treatment approach for the patient from case 2 with psoriatic lesions consisted in a gluten-free, normocaloric diet with a low glycemic index as well as limited intake of dairy, as lactose intolerance and gluten sensitivity was suspected, and no alcohol. This approach was very effective in treating the psoriatic lesions. However, inflammation indicated by high CRP was reduced only after addition of OEC. The effects of OEC on acute inflammation processes have been described in several studies. It is assumed that the anti-inflammatory effects of OEC are based, among others, on its ability to decrease neutrophil migration and secretion of proinflammatory cytokines, reduction of platelet aggregation and exhibition of fibrinolytic activities (12). The potential of OEC in reducing chronic low-grade systemic inflammation in our report is confirmed and proven by the reduction of CRP and pain assessed by VAS. These findings are in agreement with data already published and might incentivize additional efforts aiming to study more cases on this subject. The main limitation of this case report is that CRP was the only marker of low-grade systemic inflammation. New, more sensitive markers, such as ultra-sensitive (us) CRP, IL-6 and ultra-sensitive Troponin I should also be considered. A broader study should include all of them to verify the mechanisms through which enzyme therapy acts in this context.
Conclusions
The here presented results, although related to single clinical cases, show the efficacy of OEC in patients suffering from systemic chronic low-grade inflammation, pain and metabolic symptoms. The holistic treatment approaches used here, consisting of diet, exercise and OEC, led to convincing results regarding the reduction of pain, weight, asthenia and inflammation indicated by elevated CRP. The patients benefitted greatly from the combined treatment approach and showed high compliance to OEC with no adverse events reported. Further studies are needed to examine the mechanistic basis of these promising results and especially the great potential of OEC in reducing low-grade systemic inflammation which can cause great harm in the human body in the long term.
Acknowledgments
Medical writing assistance was provided by Dr. Meike Janto and Dr. Michael Wördehoff, co.medical, Berlin (Germany) on the base of the draft manuscript by the author.
Funding: This study was supported by Nestlé Health Science, Vevey (Switzerland).
Footnote
Reporting Checklist: The author has completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-45/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-22-45/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-45/coif). The author reports that medical writing assistance was provided by Dr. Meike Janto and Dr. Michael Wördehoff, Co.medical, Berlin (Germany), and this publication was sponsored by Nestlé Health Science, Vevey (Switzerland). The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the three patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
1Sold under the brand name Wobenzym® plus
References
- Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019;25:1822-32. [Crossref] [PubMed]
- Liberale L, Montecucco F, Tardif JC, et al. Inflamm-ageing: the role of inflammation in age-dependent cardiovascular disease. Eur Heart J 2020;41:2974-82. [Crossref] [PubMed]
- Soysal P, Arik F, Smith L, et al. Inflammation, Frailty and Cardiovascular Disease. Adv Exp Med Biol 2020;1216:55-64. [Crossref] [PubMed]
- Riddle MC, Herman WH. The Cost of Diabetes Care-An Elephant in the Room. Diabetes Care 2018;41:929-32. [Crossref] [PubMed]
- CDC. Health and Economic Costs of Chronic Diseases. 2022. Available online: https://www.cdc.gov/chronicdisease/about/costs/index.htm
- Leal J, Luengo-Fernández R, Gray A, et al. Economic burden of cardiovascular diseases in the enlarged European Union. Eur Heart J 2006;27:1610-9. [Crossref] [PubMed]
- Stegbauer C, Falivena C, Moreno A, et al. Costs and its drivers for diabetes mellitus type 2 patients in France and Germany: a systematic review of economic studies. BMC Health Serv Res 2020;20:1043. [Crossref] [PubMed]
- Schmid T, Brüne B. Prostanoids and Resolution of Inflammation - Beyond the Lipid-Mediator Class Switch. Front Immunol 2021;12:714042. [Crossref] [PubMed]
- Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell 2015;160:816-27. [Crossref] [PubMed]
- Calder PC, Ahluwalia N, Albers R, et al. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br J Nutr 2013;109:S1-34. [Crossref] [PubMed]
- Ranneh Y, Akim AM, Hamid HA, et al. Induction of Chronic Subclinical Systemic Inflammation in Sprague-Dawley Rats Stimulated by Intermittent Bolus Injection of Lipopolysaccharide. Arch Immunol Ther Exp (Warsz) 2019;67:385-400. [Crossref] [PubMed]
- Ueberall MA, Mueller-Schwefe GH, Wigand R, et al. Efficacy, tolerability, and safety of an oral enzyme combination vs diclofenac in osteoarthritis of the knee: results of an individual patient-level pooled reanalysis of data from six randomized controlled trials. J Pain Res 2016;9:941-61. [Crossref] [PubMed]
- Klein G, Kullich W, Schnitker J, et al. Efficacy and tolerance of an oral enzyme combination in painful osteoarthritis of the hip. A double-blind, randomised study comparing oral enzymes with non-steroidal anti-inflammatory drugs. Clin Exp Rheumatol 2006;24:25-30.
- Miller PC, Bailey SP, Barnes ME, et al. The effects of protease supplementation on skeletal muscle function and DOMS following downhill running. J Sports Sci 2004;22:365-72. [Crossref] [PubMed]
- Semwal R, Joshi SK, Semwal RB, et al. Health benefits and limitations of rutin-A natural flavonoid with high nutraceutical value. Phytochem Lett 2021;46:119-28.
- Cater JH, Wilson MR, Wyatt AR. Alpha-2-Macroglobulin, a Hypochlorite-Regulated Chaperone and Immune System Modulator. Oxid Med Cell Longev 2019;2019:5410657. [Crossref] [PubMed]
- Vandooren J, Itoh Y. Alpha-2-Macroglobulin in Inflammation, Immunity and Infections. Front Immunol 2021;12:803244. [Crossref] [PubMed]
- Henrotin YE, Michlmayr C, Rau SM, et al. Combination of Enzymes and Rutin to Manage Osteoarthritis Symptoms: Lessons from a Narrative Review of the Literature. Rheumatol Ther 2022;9:1305-27. [Crossref] [PubMed]
- Bolten WW, Glade MJ, Raum S, et al. The safety and efficacy of an enzyme combination in managing knee osteoarthritis pain in adults: a randomized, double-blind, placebo-controlled trial. Arthritis 2015;2015:251521. [Crossref] [PubMed]
- Paradis ME, Couture P, Gigleux I, et al. Impact of systemic enzyme supplementation on low-grade inflammation in humans. PharmaNutrition 2015;3:83-8.
- Leipner J, Iten F, Saller R. Therapy with proteolytic enzymes in rheumatic disorders. BioDrugs 2001;15:779-89. [Crossref] [PubMed]
- CARE case report 2013 guidelines. Available online: https://www.care-statement.org/checklist
- Ansar W, Ghosh S. Inflammation and Inflammatory Diseases, Markers, and Mediators: Role of CRP in Some Inflammatory Diseases. In: Ansar W, Ghosh S. editors. Biology of C Reactive Protein in Health and Disease. New Delhi: Springer India; 2016:67-107.
- Dinh KM, Kaspersen KA, Mikkelsen S, et al. Low-grade inflammation is negatively associated with physical Health-Related Quality of Life in healthy individuals: Results from The Danish Blood Donor Study (DBDS). PLoS One 2019;14:e0214468. [Crossref] [PubMed]
- Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375:132-40. [Crossref] [PubMed]
- Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol 2018;9:754. [Crossref] [PubMed]
- Jin X, Beguerie JR, Zhang W, et al. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:703-10. [Crossref] [PubMed]
- Saberi Hosnijeh F, Siebuhr AS, Uitterlinden AG, et al. Association between biomarkers of tissue inflammation and progression of osteoarthritis: evidence from the Rotterdam study cohort. Arthritis Res Ther 2016;18:81. [Crossref] [PubMed]
- Calder PC, Albers R, Antoine JM, et al. Inflammatory disease processes and interactions with nutrition. Br J Nutr 2009;101:S1-45. [Crossref] [PubMed]
- Stanimirovic J, Radovanovic J, Banjac K, et al. Role of C-Reactive Protein in Diabetic Inflammation. Mediators Inflamm 2022;2022:3706508. [Crossref] [PubMed]
- Kanmani S, Kwon M, Shin MK, et al. Association of C-Reactive Protein with Risk of Developing Type 2 Diabetes Mellitus, and Role of Obesity and Hypertension: A Large Population-Based Korean Cohort Study. Sci Rep 2019;9:4573. [Crossref] [PubMed]
- Vinagre I, Sánchez-Quesada JL, Sánchez-Hernández J, et al. Inflammatory biomarkers in type 2 diabetic patients: effect of glycemic control and impact of LDL subfraction phenotype. Cardiovasc Diabetol 2014;13:34. [Crossref] [PubMed]
- Bayer W. Antientzündliche Wirkungen von Omega-3-Fettsäuren. Ernährung & Medizin 2010;20:15-9.
- Kanda N, Hoashi T, Saeki H. Nutrition and Psoriasis. Int J Mol Sci 2020;21:5405. [Crossref] [PubMed]
Cite this article as: Pelosi E. Effect of oral enzyme combination, diet and exercise on chronic low-grade inflammatory conditions—a report of three cases. AME Case Rep 2023;7:7.