
A challenging case of granulomatosis with polyangiitis with cardiac involvement: a rare case report
Introduction
Granulomatosis with polyangiitis (GPA) develops over a 4–12-month time period presenting with primarily upper and lower respiratory tract symptoms in 90% of patients and 60% of patients, respectively (1). Upper respiratory symptoms include congestion, sinusitis, otitis media, mastoiditis, gingivitis, or stridor secondary to subglottic stenosis. Given the commonality of these symptoms, GPA is often not suspected until systemic symptoms develop or after multiple failed therapies. Pulmonary manifestations affect 40% of patients initially, but with progression affect 80% of patients presenting as cough, dyspnea, and hemoptysis (1). Renal involvement is present in 75% of patients and can be subclinical until the disease advances. Systemic symptoms include migratory arthritis (typically of the large joints), ocular manifestations (scleritis, episcleritis, anterior uveitis, ulcerative keratitis), neuropathy, generalized malaise, pyrexia, and weight loss. Cardiac involvement has been identified in 6–25% of patients ranging from pericarditis and myocarditis to aortitis (1).
Physical exam findings are often limited to abnormalities of the nasal septum and pulmonary exam. Typical laboratory findings include anemia, mild leukocytosis, elevated acute-phase reactants, proteinuria, and hematuria. Serum testing for antineutrophil cytoplasmic antibody (c-ANCA) aids in the clinical diagnosis with the other major pattern perinuclear antineutrophil cytoplasmic autoantibody (p-ANCA) typically associated with anti-myeloperoxidase antineutrophil cytoplasmic antibody (anti-MPO). Typically, those positive for c-ANCA also have PR3-ANCA which is highly specific (>90%) for GPA and a more severe disease course (1). The p-ANCA pattern, associated with anti-MPO, is more likely in microscopic polyangiitis; however, it can be positive in 10–25% of GPA patents (1). All positive immunofluorescence assays for ANCA should be confirmed by enzyme immunoassays including the aforementioned PR3-ANCA and anti-MPO autoantibodies to confirm the diagnosis.
Given the variability in presentation, limited physical exam findings, and the challenge with lab testing obtaining a tissue biopsy in addition to the entire clinical picture is the gold standard for diagnosis. The additional information to reach the correct diagnosis is important as the mainstay of therapy is lifelong immunosuppression with chemotherapy and biologics. Tissue histologic features include small vessel vasculitis and necrotic granulomas. Lung tissue is the best location to find the classic histologic changes of GPA as renal biopsies and nasal biopsies are less reliable. Chest computed tomography (CT) demonstrates the radiographic changes best including infiltrates, nodules, and cavitary masses. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-29/rc).
Case presentation
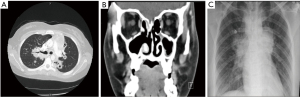
A 15-year-old woman with a history of allergic rhinitis and mild intermittent asthma was referred to the University of Oklahoma Children’s Hospital Emergency Department after having a CT chest demonstrating diffuse bilateral cavitary lesions Figure 1A. Upon further history, 6 months ago, she developed congestion and epistaxis presumed to be sinusitis after a 2-week camping trip in rural southwestern United States. Family history was significant for a maternal grandmother with systemic lupus erythematosus. She was managed conservatively initially with antihistamines and supportive care. But, after 8 weeks, she continued to progress and developed worsening cough and dyspnea despite the typical regimen for her asthma. Additionally, she further developed intermittent fevers, migratory arthralgias, and right acute otitis media (AOM). She completed a course of antibiotics, but continued to have symptoms and further developed night sweats, weight loss, and small volume hemoptysis. She was treated with further courses of antibiotics without improvement. Her fatigue and malaise progressed and she was unable to go to school or even participate in most activities of daily living. It was at this point that she went to a local emergency room and underwent the aforementioned CT scan.
Upon arrival to OU Children’s, her vital signs were significant for ongoing pyrexia with a temperature of 38.6 °C, mild tachycardia (122 beats per minute), and tachypnea (respiratory rate of 26 per minute) necessitating 2 L of supplemental O2 for increased work of breathing having SaO2 readings of within physiologic range. Physical exam was significant for boggy turbinate’s, dried blood in nasal passages, and cobble stoning of the posterior oropharynx. Pulmonary exam revealed coarse breath sounds diffusely without egophony, persistent coughing with respiratory distress. The abdominal exam was benign. Musculoskeletal exam was significant for tenderness to palpation of the hips and knees without evidence of synovitis. She had been exposed to COVID, but never had a positive test herself, and denied any tick bites during her recent camping trip. There were no known exposures to mycobacterium tuberculosis (TB), but it was noted she had been exposed to a household mold.
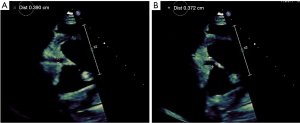
Initial laboratory studies revealed a leukocytosis of 17.3 k/mm3 with a granulocytic predominance, thrombocytosis of 698 k/mm3, and anemia with a hemoglobin of 8.3 g/dL and hematocrit of 26.7%. Furthermore, she had a sedimentation rate elevated to 119 mm/hr, C-reactive protein of 197 mg/L, ferritin of 687 ng/mL, and D-dimer of 2,184 ng/mL. Electrolyte panel was unremarkable and venous blood gas testing was unrevealing. Urinalysis had a spec gravity of 1.044 in addition to 2+ protein, 1+ ketones, 2+ blood, 5–10 white blood cell (WBC), 50–100 red blood cell (RBC), and an elevated urine microalbumin/creatinine ratio of 40. A respiratory PCR20 (polymerase chain reaction) including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) via nasopharyngeal swab was negative. Further CT imaging was significant for marked sinusitis with partial absence of the medial walls of both maxillary sinuses (Figure 1B) and plain chest radiography (CXR) (Figure 1C) was consistent with the initial CT for cavitary lesions. Given her presentation, our specialists in rheumatology and infectious disease were involved. They recommended further testing including cardiac enzymes, ANCAs, and a sputum sample with initial high suspicion for vasculitis vs. a fungal infection. Troponin T and brain natriuretic peptide (BNP) were both elevated prompting an echocardiogram which showed right coronary dilation Figure 2A,2B with noted right coronary dilation measuring 3.9 mm described as ectatic and diffusely dilated.
ANCA panel was obtained and positive at 1:80; however interestingly, both p-ANCA and c-ANCA negative. Sputum samples were negative; however, given a recent camping trip and mold exposure there was concern for possible fungal infection. As such, further fungal testing was done which was negative. All mycobacterium TB testing was negative. Bronchioalveolar lavage revealed no significant findings and cultures remained negative for bacteria including additional PCR testing for mycobacterium, but a small colony of Acremonium species growth was noted. Blood cultures remained negative, all drawn during acute febrile events. SARS-CoV-2 antibody titers were obtained and negative. Otorhinolaryngology was consulted and completed a bedside scope of the sinus cavity consistent with our CT findings demonstrating the partial absence of the medial walls of both maxillary sinuses and right middle turbinate with biopsies obtained negative for vasculitis and fungal etiologies, but positive for pseudomonas.
She was treated with a variety of antimicrobials during her stay including antibiotics and antifungals without complete resolution of symptoms. There was gradual improvement of lung cavitation’s and edema with therapy on chest CT; however, with increasing C-reactive protein (CRP) levels. Additionally, she was given intravenous immune globulin (IVIG) given the significant concern for vasculitis. Due to a lack of improvement further investigation with endobronchial ultrasound (EBUS) was done and lymph node biopsy was negative. Due to the technical difficulty of EBUS, the pulmonologist was unable to obtain a lung biopsy, a recognized diagnostic gold standard, thus further complicating diagnosis. With ongoing rises in Troponin-T and BNP cardiology suspected coronary dilation attributable to vasculitic changes, though uncommon, due to rising suspicion on our differential for GPA compared to a sole infectious etiology and no other current explainable cause for ongoing dilation with repeat echocardiography revealing the right coronary artery size increasing to 4.4 mm from 3.9 mm in diameter and recommended daily aspirin therapy with plans for outpatient follow-up.
Her symptoms of hemoptysis progressed and she was transferred to the ICU. As her infectious labs were all negative and she continued to have progressive symptoms, she was started on intravenous glucocorticoids. After initiation, she began to show improvement and was weaned off supplemental oxygen in addition to remaining afebrile. Given her improvement on steroids, all antibiotics were stopped and she continued to have improvement. Finally, our specialty lab testing for PR3-ANCA returned elevated confirming the GPA diagnosis. She started on induction cyclophosphamide and was safe for discharge with Pediatric Rheumatology follow-up.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parent for publication of this case report and accompanying images which are also declassified from patient identifiers. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In our patient significant involvement of the upper and lower respiratory tract with elevated inflammatory markers and a positive ANCA are consistent with the classic presentation of GPA. However, her age, cardiac manifestations and negative c-ANCA made her case challenging. As mentioned, GPA is typically a diagnosis in adulthood with data suggesting around 3.8% of diagnoses from a retrospective cohort study from 2006–2014 were pediatric with the average age of diagnosis close to 40 years (2).
In a thorough literature review via PubMed we were unable to find any specific cases regarding pediatric coronary dilation; however, we did find an interesting case of a cardiac mass in a 14-year-old woman with GPA (3) with most demonstrating variable cardiac involvement. The extent of variability in regard to cardiac manifestations is wide presenting in 6–44% of cases and commonly include pericarditis (the most prevalent cardiac manifestation of GPA), myocarditis, and dilated cardiomyopathy (4). Coronary aortitis or coronary artery dilatation, are rarely identified, and in our review, no previous cases of coronary artery dilatation as a manifestation of GPA were identified in the adolescent population. If found, coronary artery dilation presented in older patients and manifested as myocardial infarction (5); however, in our patient aside from the previously mentioned troponin and BNP elevation there was no evidence of infarction on ECG or impaired systolic function on echocardiogram without regional wall motion. While this finding of coronary dilation is not a known complication of GPA, it does emphasize that evaluation of potential cardiovascular complications is indicated. The suspected mechanism of such coronary dilation include immune deposits, neutrophilic vasculitis, necrosis, and micro abscesses with proteolytic enzyme degradation of the endothecium triggering further vasculopathic cascade (6). It is imperative to note that cardiac complications can have an acute presentation such as acute ST elevated myocardial infarction, thus further providing evidence for the need of evaluation (7). We do know other systemic vasculitic processes (for example mucocutaneous lymph node syndrome) are historically associated with coronary dilation and given this it seems likely that there may be a significant portion that remain unidentified if not prompted by clinical or laboratory findings like in our patient.
In regard to the seemingly contradictory lab work, it seems that there are a percentage of patients who may not necessarily have a positive c-ANCA and still test positive for PR3-ANCA and have clinical systemic vasculitis. The c-ANCA and p-ANCA are tested via indirect immunofluorescent assays typically used to help guide further testing for definitive antibodies PR3-ANCA and anti-MPO respectively by enzyme-linked immunosorbent assays (ELISA) (8). We know that a positive c-ANCA on its own is not diagnostic of GPA and requires consideration of the clinical findings, history, and further confirmatory testing such as the ELISA and tissue biopsy. Given the severity of disease in our case it seems that when the clinical history and physical exam findings are suggestive of a vasculitic process one should always consider additional testing with ELISA regardless of the IIF results for ANCA which appears to be contradictory to studies that indicate the utility of obtaining both in diagnosing clinically significant vasculitis is not helpful., with the understanding that clinically we agree it is likely of no benefit without strong clinical suspicion (9).
Conclusions
GPA is a rare, but clinically devastating disease which can masquerade as a multitude of common disease states classically of infectious etiology. As in our case if left untreated can have significant systemic effects with marked pathology of the pulmonary, renal, and cardiovascular systems. Timely consideration of systemic vasculitis is important in these patients and while uncommon, if the clinical picture is suspicious further work up should be obtained even in pediatric patients as they make up a small, but significant portion of those affected and typically with more severe clinical progression. We want to emphasize that while not classically listed in the literature, special attention to evaluating for cardiovascular pathology such as coronary involvement is prudent. Finally, while classically associated with c-ANCA patients do not necessarily have to have a specific ANCA subtype to prompt further confirmatory testing with PR3-ANCA and anti-MPO if the diagnosis is considered.
Acknowledgments
Funding: The publication fee of this study was supported by the Department of Pediatric Rheumatology at Oklahoma University Health Sciences Center at the University of Oklahoma Children’s Hospital.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-29/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-22-29/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-29/coif). AS reports that the publication fee of this study was supported by the Department of Pediatric Rheumatology at Oklahoma University Health Sciences Center at the OU Children’s Hospital. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parent for publication of this case report and accompanying images which are also declassified from patient identifiers. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yazdany J, Manno RL. Granulomatosis with Polyangiitis. In: Papadakis MA, McPhee SJ, Rabow MW, McQuaid KR. eds. Current Medical Diagnosis & Treatment 2022. McGraw Hill; 2022. Accessed February 25, 2022. Available online: https://accessmedicine-mhmedical-com.webproxy2.ouhsc.edu/content.aspx?bookid=3081§ionid=258968221
- Panupattanapong S, Stwalley DL, White AJ, et al. Epidemiology and Outcomes of Granulomatosis With Polyangiitis in Pediatric and Working-Age Adult Populations In the United States: Analysis of a Large National Claims Database. Arthritis Rheumatol 2018;70:2067-76. [Crossref] [PubMed]
- Harris JG, Salvay DM, Klein-Gitelman MS. Asymptomatic intracardiac mass in a 14-year-old girl with granulomatosis with polyangiitis: Case report. Pediatr Rheumatol Online J 2012;10:9. [Crossref] [PubMed]
- Shanahan EM, Sheahan K, McDonald K, et al. Dilated cardiomyopathy in Wegener's granulomatosis. Rheumatology (Oxford) 1999;38:1164-6. [Crossref] [PubMed]
- Cocco G, Gasparyan AY. Myocardial ischemia in Wegener's granulomatosis: coronary atherosclerosis versus vasculitis. Open Cardiovasc Med J 2010;4:57-62. [Crossref] [PubMed]
- McGeoch L, Carette S, Cuthbertson D, et al. Cardiac Involvement in Granulomatosis with Polyangiitis. J Rheumatol 2015;42:1209-12. [Crossref] [PubMed]
- Lazarus MN, Khurana R, Sethi AS, et al. Wegener's granulomatosis presenting with an acute ST-elevation myocardial infarct (STEMI). Rheumatology (Oxford) 2006;45:916-8. [Crossref] [PubMed]
- Wiik A. What you should know about PR3-ANCA. An introduction. Arthritis Res 2000;2:252-4. [Crossref] [PubMed]
- Rao DA, Wei K, Merola JF, et al. Myeloperoxidase-antineutrophil Cytoplasmic Antibodies (MPO-ANCA) and Proteinase 3-ANCA without Immunofluorescent ANCA Found by Routine Clinical Testing. J Rheumatol 2015;42:847-52. [Crossref] [PubMed]
Cite this article as: Shelton A, Parikh S, Mims C, Quintero-Del-Rio A. A challenging case of granulomatosis with polyangiitis with cardiac involvement: a rare case report. AME Case Rep 2023;7:8.


