Parsonage Turner syndrome after cervical trauma and COVID-19 infection: a case report and review of the literature
Introduction
Parsonage Turner syndrome, otherwise known as Neuralgic Amyotrophy, is an uncommon condition with an incidence of 1–3 cases in 100,000 people (1). This condition can occur at any age but most commonly affects young adults and has a male predilection (2). Although etiology is poorly understood, it is thought that Parsonage Turner syndrome is an immune-mediated plexopathy (3). Parsonage Turner syndrome is typically characterized by acute onset severe shoulder pain which may extend to the proximal and distal upper limb, including the hand. The pain is usually described as sharp aching or burning, often unilateral and can be position dependent. Pain usually subsides within 1–2 weeks followed by muscle weakness and atrophy that may involve any distribution of the brachial plexus, predominantly affecting the roots (2). While clinical history and physical examination are key, electrodiagnostic studies and imaging may corroborate diagnosis. Full functional recovery can be expected in up to 89% of patients after 3 years (4).
The COVID-19 global pandemic has brought to light the otherwise relatively rare condition of Parsonage Turner syndrome. Prior reports of Parsonage Turner associated with COVID-19 have been reported around the world. We report a rare case of Parsonage Turner syndrome after posterior cervical spinal surgery with postoperative COVID-19 infection and provide a discussion on the presentation and diagnostic work up. Most reported cases of Parsonage Turner syndrome have a known associated risk factor including surgery, trauma and increasingly common viral infections including COVID-19. However, this case is unique in that this patient had several concurrent risk factors. He had bilateral Parsonage Turner syndrome which had slightly different onset. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-33/rc).
Case presentation
A 62-year-old man with remote shoulder surgery for clavicle fracture and remote anterior cervical spine fusion presented after a fall at home where he lost consciousness. No cervical spine fracture was noted initially on computed tomography (CT) scan per radiology report and the patient was discharged with a Philadelphia cervical collar from the emergency room for neck pain.
Ten days later, the patient presented to clinic with excruciating neck pain and left radicular arm pain with paresthesia. He had no myelopathic symptoms on exam and he had full motor strength in both upper and lower extremities with normal reflex. A review of systems was negative for any other symptoms. Standing upright plain films of the cervical spine showed a right C4-C5 facet fracture with focal cervical kyphosis. He also had evidence of previous C5-C7 anterior cervical fusion. Due to concern for an unstable fracture, patient was admitted to the hospital for advanced imaging and surgical fixation of his cervical spine. A preoperative magnetic resonance imaging (MRI) was obtained due to patient’s left arm pain and concern for possible nerve impingement, which revealed severe C3-C4 spinal canal, lateral recess, and foraminal stenosis secondary to disc protrusion and uncovertebral spurring.
The patient underwent a C2-T2 posterior fusion with instrumentation and C3-C4 laminectomy. Electrodiagnostic testing for somatosensory and motor evoked potential during surgery were stable throughout and the patient tolerated surgery well. On post-operative day 1, the patient complained of right shoulder pain and on day 2, he noted left sided progressive weakness and numbness in his upper extremity. Magnetic resonance imaging (MRI) and CT scan of the cervical spine was completed postoperatively which did not show any new nerve compression, spinal cord compression or hardware failure or misplacement. Physical exam revealed left sided ulnar claw hand and sensitivity over the ulnar aspect of the volar wrist. He was discharged on post-operative day 4 and instructed to follow up for electrodiagnostic testing.
Three days after discharge, he presented to the emergency department for persistent pain in his right shoulder. He tested positive for COVID-19 via polymerase chain reaction (PCR). Notably, his preoperative PCR test was negative for COVID-19 the day before his surgery. He was discharged with a prescription for gabapentin 600 mg, cyclobenzaprine 10 mg every 8 hours and a nonsteroidal anti-inflammatory drug (NSAID). His COVID-19 symptoms of cough and progressive dyspnea worsened, and he presented to the emergency department again. He had worsening non-productive cough, fevers, chills, muscle aches and shortness of breath. He was admitted for acute hypoxic respiratory failure, for which he had a 6-day hospital stay. He was treated with a 5-day course of Remdesivir but not mechanically ventilated nor placed in prone position for an extended period of time.
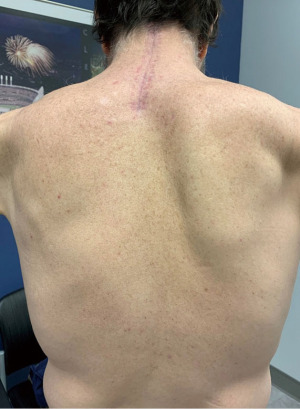
At his 8-week post-operative follow-up visit, he was found to have right scapula winging (Figure 1). Physical and occupational therapy was recommended for his right shoulder and left-hand symptoms, respectively. He had been treated with gabapentin and his pain was much improved. Electrodiagnostic testing was ordered, which the patient did not complete. Twelve weeks after his surgery, pain had resolved, yet he still had right-sided winged scapula and weakness. After extensively working with physical therapy, he had full range of motion in his left hand but had noticeable thenar atrophy.
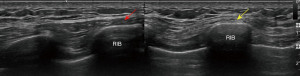
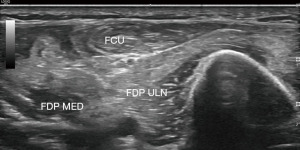
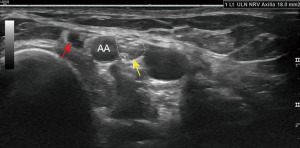
At 20 weeks after surgery, the patient underwent electromyography and neuromuscular ultrasound study of his upper extremities. There was evidence of prominent denervation atrophy affecting the right serratus anterior, consistent with a chronic long thoracic nerve palsy (Figure 2). Muscles innervated by the ulnar and median nerve showed signs of denervation atrophy (Figure 3). The left upper extremity showed prominent evidence of denervation atrophy affecting the ulnar innervated muscles, especially the flexor carpi ulnaris and flexor digitorum profundus. Median innervated flexor digitorum profundus also appeared to be affected. The ulnar nerve was normal in the forearm, at the elbow and upper arm, but unequivocally enlarged at the axilla (Figure 4). Findings were consistent with Parsonage Turner syndrome. He still reported the lingering COVID-19 symptoms of fatigue and lethargy. Patient was recommended to continue working with physical and occupational therapy. About 1.5 years after surgery, he regained his strength with 5 out of 5 motor strength in bilateral upper extremities.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was waived by our institutional review board and approval was granted for publication of this case report and accompanying images. A copy of the approval is available for review by the editorial office of this journal.
Discussion
The global pandemic caused by COVID-19 caused a rise in reported cases of Parsonage Turner syndrome. A literature review of COVID-19-related Parsonage Turner syndrome included case reports and case series from Germany, Italy, Chile, Kuwait, and the United States of America. Although the review revealed rare presentations of Parsonage Turner syndrome, it was found that overall, similar management was employed. Diagnosing COVID-19 related Parsonage Turner syndrome is diagnostic. However, electromyographic studies, magnetic resonance and ultrasonographic studies may be used as well. Steroids and NSAIDs are used in the acute setting in the management of COVID-19 related Parsonage Turner syndrome.
Although there have been some prior reports, bilateral Parsonage Turner syndrome is a very rare presentation (5-9). Unlike most reported cases of Parsonage Turner syndrome, the patient in this report had a few possible triggers that lead to the development of bilateral parsonage turner syndrome. He developed left sided Parsonage Turner syndrome symptoms that predate his spine surgery, and his right sided symptoms came on after spine surgery. Although rare, trauma is a recognized potential trigger for Parsonage Turner syndrome. In a description of the clinical spectrum of Parsonage Turner syndrome in 246 patients by van Alfen et al., 4.3% had antecedent trauma (4). Local trauma was present in 43 out of 45 cases of brachial plexus neuropathy with known cause in a different study (1). The patient in this report had a fall that led to a cervical fracture that could have served as the initial trigger for the development of Parsonage Turner Syndrome. Cases with a known trigger typically present within two weeks (1). Ten days after initial presentation, the patient complained of pain and paresthesia in the left arm. However, the patient herein had bilateral Parsonage Turner syndrome, seemingly of disparate onset. He only noted right sided symptoms 11 days after initial trauma which was one day after his surgery.
As the COVID-19 (SARS-CoV-2) global pandemic spread throughout the world, there was an increase in reports of rare neurologic conditions of the peripheral nervous system (10). Sometime during the preoperative or perioperative period in the hospital, the patient was infected with COVID-19. His left sided symptoms began before he had any COVID-19 symptoms or tested positive for COVID-19. However, it is uncertain if this infection preceded his right sided symptoms as infection can occur about a week prior to a positive test for COVID-19. To our knowledge, there have only been a few reports of COVID-19 associated Parsonage Turner syndrome to date (Table 1) (11-19). Typically, patients develop Parsonage Turner syndrome symptoms within 14 days on COVID-19 infection. There is significant variability in COVID-19 related presentation of Parsonage Turner syndrome (11). One report presented a patient with sole median nerve involvement which was experienced during initial two-week quarantine (11). Cacciavillani et al. report the case of a patient who presented with purely sensory Parsonage Turner syndrome of lateral antebrachial cutaneous distribution without any clinical or electrophysiologic signs of motor involvement 12 days after diagnosis of COVID-19 (16). In a report by Mitry et al., an unconfirmed case of COVID-19 presented with a 3-month history of Parsonage Turner syndrome symptoms after the resolution of acute respiratory illness (17).
Table 1
| Paper | Patient age (years)/sex/dominant hand | PTS symptoms | Symptom onset with respect to COVID-19 positive test | Potential triggers | Diagnostic (timing) findings | Clinical findings | Management | Follow up |
|---|---|---|---|---|---|---|---|---|
| Siepmann et al., 2020 (11) | 52/ M/R | Right shoulder pain with gradual shift to forearm and hand, paresthesia involving index and long fingers | First 2 weeks | COVID infection | NCS (4 weeks): motor axonal neuropathy of median nerve; EMG (4 weeks): decrease in motor unit recruitment in APB, OP. No motor unit activity in FPL; MRI: edema of the cervical spine, inflammatory contrast enhancement of right distal median nerve; nerve US: substantial swelling of the R median nerve from brachial plexus to wrist | Weakness of FDP, FPL, AP, OP, median nerve hyperesthesia | Oral prednisolone (1 mg/kg body weight once daily) for 7 d followed by tapering and discontinuation by day 17 | Partial pain relief at 7 and 14 days. No improvement of pain |
| Ismail et al., 2021 (12) | 32/M/R | Left shoulder pain aggravated by touch and movement. Pain involved the right shoulder 1 week later which progressed to forearms. Proximal weakness after 2 weeks and bilateral hand numbness with sensory loss over the left shoulder and radial forearm | Within 1 week | COVID-19 infection; COVID-19 hospitalization | (5 weeks) EDX: sensory and motor axonal low amplitude of compound motor action potentials of musculocutaneous, axillary, and suprascapular nerves on both sides, and long thoracic and anterior interosseous nerve on the left side, with patchy denervation; MRI: hyperintense T2-weighted signal of supra- and infraspinatus muscles, indicating intramuscular edema, with normal cervical spine and both brachial plexuses | Left-sided winged-scapula, weakness of shoulder abduction and flexion bilaterally and IP joints of the thumb and index on the left side. Right-sided weakness on elbow flexion. Minimal atrophy bilaterally. Reduced sensation over both shoulders | Acetaminophen initially, pregabalin 300 mg/day, tramadol hydrochloride 50 mg twice daily, and local injection of steroids and lidocaine in both shoulders initially. Subsequently methylprednisolone 1,000 mg/d for 5 days (stopped due to dermatological side effects), immunoglobulins (IVIg) in a dose of 25 g/d for 5 days | Partial relief of pain with no improvement in muscle power after 8 weeks of symptom onset |
| Coll et al., 2021 (13) | 63/M | Pain and disability in right shoulder | 4 weeks | COVID-19 infection; COVID-19 related mechanical ventilation resuscitation for 6 weeks by intubation and tracheotomy | NCS (21 weeks): normal latencies with marked reduction of compound action potentials amplitude of R upper and lower trapezius muscles; EMG: reduced interference patterns and denervation signs in upper and lower trapezius muscles; MRI (7 months): no inflammatory modification of cranial nerve XI | Active and passive limitation of shoulder ROM, and muscular atrophy of upper trapezius muscle and supra-infra-spinous fossae; paresthesia in the ulnar area of both forearms. Mildly winged scapula and altered scapulothoracic rhythm | NS | NS |
| Coll et al., 2021 (13) | 74/M | Pain and disability of left shoulder | 1 week | Mechanical ventilation resuscitation for 5 weeks by intubation | NCS (17 weeks): normal latencies with marked reduced compound action potentials amplitude of left upper and lower trapezius muscles; EMG: reduced interference patterns and denervation signs in upper and lower trapezius muscles; MRI (21 weeks): muscular atrophy of left trapezius | Muscular atrophy of upper trapezius muscle and supra-infra-spinous fossae; active and passive shoulder ROM were limited in the three spatial planes; shoulder weakness a mild winged scapula | NS | NS |
| Alvarado et al., 2021 (14) | 38/M | Pain in bilateral shoulders with burning and tingling sensation, which disrupted sleep and partially remitted with conventional analgesics. Subsequent muscle weakness in shoulders | NS | COVID-19 infection; COVID-19 related mechanical ventilation prone position ventilation | EDX: left brachial plexopathy and absence of sensory response in the lateral antebrachial cutaneous nerve, acute motor axon loss in the trapezius, deltoid, and serratus anterior. MRI: edema in both infraspinatus muscles and tendinosis of both supraspinatus muscles | Bilateral atrophy of the deltoid, supraspinatus, and infraspinatus muscles, right scapular winging, and hypoesthesia in the deltoid | NSAIDs combined with long-acting opioids and prednisone | NS |
| Alvarez et al., 2021 (15) | 46/F | Pain and weakness in the left shoulder | 6 weeks | COVID-19 infection; mechanical intubation for 23 days; prone positioning intermittently | MRI: moderate edema of the deltoid, teres major, teres minor, and the latissimus; EDX (2–3 months): mild left median neuropathy at the wrist and motor unit recruitment pattern consistent with a chronic left upper trunk plexopathy with reinnervation | positive sulcus sign, atrophy of the left shoulder, and tenderness of the subacromial region. limited active ROM by pain 4/5 left shoulder abduction and extension. Positive left Hawkins and empty can tests. left Hoffman reflex. 4/5 left hip flexion. Diminished sensation to crude touch in the left lateral thigh. | Meloxicam | Strength and Hoffman sign had normalized by 3 months |
| Cacciavillani et al., 2021 (16) | 52/M | Pain in the left wrist and upper limb in the distribution of the lateral antebrachial cutaneous nerve followed by hypoesthesia and dysesthesia | 12 days | COVID-19 infection | NCS: (5 weeks) reduced sensory nerve action potential amplitude of the left lateral antebrachial cutaneous nerve | Hypoesthesia and dysesthesia in the same distribution. | NS | Persistent hypoesthesia and dysesthesia 6 weeks after onset |
| Mitry et al., 2021 (17) | 17/F | Constant pain exacerbated by movement | 17 weeks | COVID-19 infection; post COVID-19 hyperinflammatory syndrome such as multisystem inflammatory syndrome in children (MIS-C) | Full ROM, limited by pain | MRI: increased T2 signal of the supraspinatus, infraspinatus, teres minor, teres major, and trapezius muscles | Oral steroids | NS |
| Ansari et al., 2022 (18) | 55/M | Pain in his left scapular region, followed by proximal left upper limb weakness; pain along cervical spine and medial scapula | 17 days | COVID-19 infection | MRI: slight C3-C4 herniation, plexus MRI normal; EMG: spontaneous activity in left biceps, deltoid, supraspinatus and rhomboid muscles, upper trunk brachial amyotrophy | Reduced elevation, abduction and external formation of left shoulder; proximal left upper limb muscle weakness with intact distal muscle strength; absent left biceps deep tendon reflexes; sensory deficit in lateral arm and forearm | Oral prednisolone (25 mg) for 3 weeks followed by tapering | 8 weeks: normal shoulder exam without pain or functional impairment |
| Díaz et al., 2021 (19) | 36/M/L | Neuropathic pain in right shoulder without radicular distribution, progressive weakness in right upper limb | 2 weeks following development of symptoms suggestive of COVD-19 infection | COVID-19 | MRI: right shoulder, supraspinatus and infraspinatus intramuscular hyperintensity and global atrophy of periscapular musculature; EDX: signs of subacute and severe degree of right brachial plexopathy with elements of denervation | Muscular atrophy of supraspinatus and infraspinatus fossa, deltoids and biceps, preserved passive mobility, decreased force in anterior elevation, abduction, external rotation and internal rotation, no scapular dyskinesis for statis and dynamic evaluation; scapular balance <5 degrees, reflexes normal | Neuromodulator drugs (pregabalin 75 mg bid for 4 months), kinesiological treatment, assisted passive and active mobility exercises of right limb, mobility exercises and isometric activation of stabilizing muscles, exercises to strengthen elbow and shoulder flexors and right shoulder abductors | 6 months: discharged symptom-free |
F, female; M, male; NCS, nerve conduction study; EDX, electrodiagnostic study; NS, not specified; EMG, electromyography; APB, abductor pollicis brevis; OP, opponens pollicis; FPL, flexor pollicis longus; MRI, magnetic resonance imaging; US ultrasound; FDP, flexor digitorum profundus; AP, adductor pollicis; IP, interphalangeal; ROM, range of motion; NSAID, nonsteroidal anti-inflammatory drug.
COVID-19-related Parsonage Turner syndrome may present bilaterally and may be due to trauma related to management of COVID-19. In traumatic COVID Parsonage Turner syndrome, patients are ventilated mechanically and placed in prone position intermittently for oxygenation (20). Such trauma has been reported to be a potential trigger for Parsonage Turner syndrome. In some of the reported COVID-19 associated Parsonage Turner syndrome cases, trauma may be an unrecognized triggering. Alvarez et al. present a case of atraumatic Parsonage Turner syndrome despite possible traumatic triggers (15).
A thorough physical exam is necessary in those suspected of Parsonage Turner syndrome as it is a clinical diagnosis. Electrodiagnostic testing localizes to the brachial plexus and excludes other radiculopathies or neuropathies. Similarly, imaging establishes diagnosis and excludes differential diagnoses. Adhesive capsulitis, impingement syndromes, rotator cuff or labral tears can all be diagnosed by MRI. Scalf et al. reported intramuscular denervation changes in 26 Parsonage Turner syndrome patients (21). Furthermore, ultrasonography has been used to aid in the diagnosis of Parsonage Turner syndrome. One study found that ultrasonographic testing had an overall sensitivity of 74% among 53 patients (22). MRI and ultrasound have shown hourglass constrictions with marked swelling preceding caliber constriction of the nerves (21,23). Ultrasonographic findings of fascicular entwinement in this report are suggestive of Parsonage Turner syndrome (Video 1).
Here we report a case of bilateral Parsonage Turner syndrome after posterior cervical spinal fusion for a displaced right C4-C5 facet fracture in the setting of COVID-19 infection. The timeline is most supportive of his cervical spine trauma as the likely trigger. However, the differential onset of symptoms in each arm and the bilateral nature of his condition suggests involvement of another trigger. Surgery may have precipitated his latter right-sided symptoms. It is uncertain exactly when this patient was infected with COVID-19. Therefore, it was important to consider as a potential trigger especially in the context of the growing number of COVID-19-related Parsonage Turner syndrome reports.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-33/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-22-33/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-33/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was waived by our institutional review board and approval was granted for publication of this case report and accompanying images. A copy of the approval is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Beghi E, Kurland LT, Mulder DW, et al. Brachial plexus neuropathy in the population of Rochester, Minnesota, 1970-1981. Ann Neurol 1985;18:320-3. [Crossref] [PubMed]
- van Alfen N. Clinical and pathophysiological concepts of neuralgic amyotrophy. Nat Rev Neurol 2011;7:315-22. [Crossref] [PubMed]
- Suarez GA, Giannini C, Bosch EP, et al. Immune brachial plexus neuropathy: suggestive evidence for an inflammatory-immune pathogenesis. Neurology 1996;46:559-61. [Crossref] [PubMed]
- van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain 2006;129:438-50. [Crossref] [PubMed]
- Feinberg JH, Doward DA, Gonsalves A. Cervical radiculopathy vs Parsonage-Turner syndrome: a case report. HSS J 2007;3:106-11. [Crossref] [PubMed]
- Gaskin CM, Helms CA. Parsonage-Turner syndrome: MR imaging findings and clinical information of 27 patients. Radiology 2006;240:501-7. [Crossref] [PubMed]
- Lindgren B, Rivers D, Clark J. Bilateral Parsonage-Turner Syndrome After Initial Unilateral Presentation: A Case Report. Cureus 2019;11:e6422. [Crossref] [PubMed]
- Ohta R, Shimabukuro A. Parsonage-Turner syndrome in a patient with bilateral shoulder pain: A case report. J Rural Med 2017;12:135-8. [Crossref] [PubMed]
- Van Tongel A, Schreurs M, Bruyninckx F, et al. Bilateral Parsonage-Turner syndrome with unilateral brachialis muscle wasting: a case report. J Shoulder Elbow Surg 2010;19:e14-6. [Crossref] [PubMed]
- Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683-90. [Crossref] [PubMed]
- Siepmann T, Kitzler HH, Lueck C, et al. Neuralgic amyotrophy following infection with SARS-CoV-2. Muscle Nerve 2020;62:E68-70. [Crossref] [PubMed]
- Ismail II, Abdelnabi EA, Al-Hashel JY, et al. Neuralgic amyotrophy associated with COVID-19 infection: a case report and review of the literature. Neurol Sci 2021;42:2161-5. [Crossref] [PubMed]
- Coll C, Tessier M, Vandendries C, et al. Neuralgic amyotrophy and COVID-19 infection: 2 cases of spinal accessory nerve palsy. Joint Bone Spine 2021;88:105196. [Crossref] [PubMed]
- Alvarado M, Lin-Miao Y, Carrillo-Arolas M. Parsonage-Turner syndrome post-infection by SARS-CoV-2: a case report. Neurologia 2021;36:568-71. (Engl Ed). [Crossref] [PubMed]
- Alvarez A, Amirianfar E, Mason MC, et al. Extended Neuralgic Amyotrophy Syndrome in a Confirmed COVID-19 Patient After Intensive Care Unit and Inpatient Rehabilitation Stay. Am J Phys Med Rehabil 2021;100:733-6. [Crossref] [PubMed]
- Cacciavillani M, Salvalaggio A, Briani C. Pure sensory neuralgic amyotrophy in COVID-19 infection. Muscle Nerve 2021;63:E7-8. [Crossref] [PubMed]
- Mitry MA, Collins LK, Kazam JJ, et al. Parsonage-turner syndrome associated with SARS-CoV2 (COVID-19) infection. Clin Imaging 2021;72:8-10. [Crossref] [PubMed]
- Ansari B, Eishi Oskouei A, Moeinzadeh F. Parsonage-Turner Syndrome following COVID-19 Infection: A Rare and Unique Case. Adv Biomed Res 2022;11:7. [PubMed]
- Díaz C, Contreras JJ, Muñoz M, et al. Parsonage-Turner syndrome association with SARS-CoV-2 infection. JSES Rev Rep Tech 2021;1:252-6. [PubMed]
- Le MQ, Rosales R, Shapiro LT, et al. The Down Side of Prone Positioning: The Case of a Coronavirus 2019 Survivor. Am J Phys Med Rehabil 2020;99:870-2. [Crossref] [PubMed]
- Scalf RE, Wenger DE, Frick MA, et al. MRI findings of 26 patients with Parsonage-Turner syndrome. AJR Am J Roentgenol 2007;189:W39-44. [Crossref] [PubMed]
- ArÁnyi Z. Ultrasonography in neuralgic amyotrophy: Sensitivity, spectrum of findings, and clinical correlations. Muscle Nerve 2017;56:1054-62. [Crossref] [PubMed]
- Sunagawa T, Nakashima Y, Shinomiya R, et al. Correlation between "hourglass-like fascicular constriction" and idiopathic anterior interosseous nerve palsy. Muscle Nerve 2017;55:508-12. [Crossref] [PubMed]
Cite this article as: Ahorukomeye P, Pennacchio CA, Preston DC, Cheng CW. Parsonage Turner syndrome after cervical trauma and COVID-19 infection: a case report and review of the literature. AME Case Rep 2022;6:37.