
Malignant anaplastic meningioma in neurofibromatosis type 1 patient: a rare case report
Introduction
Grade 3 anaplastic meningiomas account for 1–3% of all meningiomas, and they are more aggressive with a worse clinical course (1). They have an increased tendency for recurrence and higher disease-specific mortality (1). The most common genetic syndrome associated with the risk of developing a meningioma is neurofibromatosis type 2 (NF-2). However, neurofibromatosis type 1 (NF-1) is associated with a high risk of developing low-grade and high-grade gliomas (2). Here, we report a rare case of NF-1 simultaneously presented with a malignant anaplastic meningioma, which is classified as grade 3, based on the 2021 World Health Organization (WHO) classification of tumors of the central nervous system (CNS) (3). The mainstay of management of grade 3 meningiomas is surgical resection followed by radiotherapy. The use of chemotherapy in grade 3 meningiomas is still experimental and preserved for recurrent grade 3 meningiomas that are unsuitable for more surgery or radiotherapy. However, many reports showed few benefits (1,4). Our patient presented with a high-grade meningioma in which a chemotherapeutic agent [temozolomide (TMZ)] was added to her treatment course. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-8/rc).
Case presentation
A 25-year-old left-handed female, a known case of NF-1, confirmed clinically, presented initially to her local hospital with focal seizures. Upon radiological investigations, she was found to have an extra-axial lesion in the left cerebral convexity (Figure 1). The lesion was resected entirely, and pathology reported it as a grade 3 anaplastic meningioma.
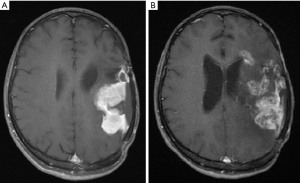
Two months later, the patient was referred to our tertiary hospital for adjuvant radiotherapy. On physical examination, she was found to have right-sided spastic hemiparesis and features of NF-1, including more than six café-au-lait lesions on her back, chest, and upper limbs. There was an axillary freckling, three cutaneous neurofibromas on her back, and a plexiform neurofibroma on her right flank area. She had a history of a first-degree relative with NF-1. Further imaging revealed a local recurrence of the tumor (Figure 1A).
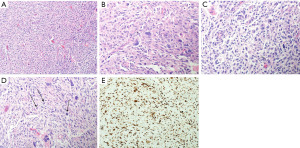
The patient underwent a second surgical debulking of the tumor. The pathological analysis confirmed a WHO grade 3 anaplastic meningioma (Figure 2) with a high Ki-67 proliferative index of up to 30% and negative progesterone receptors. Following surgical resection, the patient’s hemiparesis improved. A few weeks later, she received adjuvant radiotherapy with a total dose of (60 Gy in 30 fractions).
Approximately 1 year later, the patient was re-admitted with further recurrence, and a third tumor debulking was carried out. Two months later, the patient presented to the clinic with a one week history of right hand and right facial weakness. A brain MRI was performed, which revealed a local recurrence of the tumor. The patient underwent a fourth tumor debulking operation.
Her case was then discussed in our neuro-oncology tumor board as an aggressive disease with multiple recurrences over a short period of time. The decision was made to go for palliative re-irradiation (37.5 Gy in 15 fractions) plus the addition of a chemotherapeutic agent (TMZ) (for six cycles), without the need for further operations. The patient received only three cycles of TMZ due to its side effects. At this stage, she was referred to a palliative care clinic.
Her latest brain MRI (almost 42 months from diagnosis) showed a multifocal recurrence of the tumor involving the left insular, parietal lobe, and left frontal horn (Figure 1B). Her overall survival was 50 months. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). We could not obtain written informed consent from the patient as she passed away a few years ago. An attempt was made to contact the patient’s family, but this was unsuccessful. Therefore, we followed our institution’s guidelines for case reports and reported the case after removing all personal health information, to ensure that the patient could not be identified by the history, figures, or pathology included in the report.
Discussion
One of the most common primary CNS tumors is meningiomas. They account for 37.6% of all primary CNS tumors and 53.3% of benign CNS tumors (5). The overall survival and the risk of recurrence of meningiomas are based on the 2021 WHO grading system (3). The higher the grade, i.e., grade 2 or 3, the more aggressive meningiomas will become. Therefore, this grading system has a significant role in the management strategy (5). The 10-year overall survival rate of WHO grade 1 is 83.7%, for grade 2 is 53%, and 0% for grade 3, regardless of the aggressive therapeutic interventions (5).
There are various risk factors for developing meningiomas, such as; exposure to ionizing radiation or pesticides/herbicides; which are occupational hazards, obesity, hormones, and genetics (5). The most common genetic syndrome associated with the risk of developing a meningioma, whether intracranial or spinal, is NF-2 (6). Furthermore, the risk of developing meningioma is almost close to 80% by the age of 70 years in NF-2 patients of both genders (7). In contrast, NF-1 patients are at high risk of developing low-grade and high-grade gliomas (2). NF-1 is diagnosed clinically based on the National Institutes of Health (NIH) criteria which require the presence of at least two or more features of NF-1 (8).
Grade 3 anaplastic meningioma accounts for 1–3% of all meningiomas, and they are more aggressive with a worse clinical course. They have an increased tendency for recurrence and higher disease-specific mortality (1). According to WHO, they are diagnosed with either of two criteria: (I) greater than or equal to 20 mitoses per 10 high power field (HPF) and/or (II) frank anaplasia (sarcoma, carcinoma, or melanoma-like histology) (1).
A high Ki-67 index is associated with tumor recurrence and poor prognosis. Anaplastic meningiomas acquire this common feature of a high Ki-67 index (9). The Ki-67 proliferation index of >4% has an increased recurrence risk, and >20% has an increased mortality risk (5). Furthermore, progesterone receptors (PR) are expressed in almost 50–80% of meningiomas, with an inverse relationship with the meningiomas’ WHO grade. Aggressive meningiomas with increased recurrence rates tend to have a loss or absence of progesterone receptors. In contrast, meningiomas with a more favorable prognosis tend to have higher levels of progesterone receptors. Almost all WHO grade 3 meningiomas exhibit an absence of PR expression (10).
The mainstay of management of symptomatic meningiomas is surgical resection. The most significant modifiable predictor of local control and tumor recurrence is the extent of tumor resection, regardless of tumor grade or other prognostic factors (1). Therefore, the aim of surgery is gross total resection (GTR), but that could be difficult to attain in certain circumstances such as the location of the tumor, the proximity to venous sinuses, neurovascular tissue involvement, or brain invasion that may limit the extent of resection (5). In such cases, the most appropriate approach is the maximum safe resection with subsequent postoperative adjuvant radiotherapy. Moreover, even after a complete GTR of WHO grade 3 meningiomas, they will still have a high risk of recurrence after 5 years postoperatively by 50–80% (5). Therefore, it is recommended for grade 3 anaplastic meningiomas, regardless of the extent of tumor resection, to receive an adjuvant fractionated radiotherapy of at least 54 Gy given in 1.8–2.0 Gy per fraction (5). However, doses can range from 50 to 70 Gy, as higher doses may improve patient outcomes based on evidence from several retrospective studies (1).
Recurrent meningiomas often become refractory to standard radiation and surgical therapies (1). In addition, it has been reported that grade 3 anaplastic meningiomas have a rapid recurrence postoperatively after removing the primary tumor in almost less than 2 months period (9).
Due to the rapidly progressive and recurrent nature of grade 3 meningiomas and the failure to respond to surgery and radiotherapy, a systemic therapy (TMZ) was pursued in this case based on reports from the literature. It has been demonstrated the limited clinical efficacy in treating meningiomas with chemotherapy or other systemic therapies, as they are used after exhausting the standard options in treating refractory meningioma that keeps recurring or progressing after surgery and adjuvant radiotherapy (4). TMZ is a cytotoxic alkylating agent that acts on cell nucleus and inhibits tumor cells proliferation by inducing cellular apoptosis (4).
In the phase II prospective study, TMZ has been used in the treatment-resistant recurrent meningioma, where patients have been previously operated on and irradiated; they have failed to demonstrate progression-free survival at 6 months. As a result, and based on this study, TMZ appears not to have clinical efficacy in treating recurrent meningiomas, like many other cytotoxic treatments (11). Multiple approaches were also investigated, such as targeting estrogen and progesterone receptors, which failed to demonstrate a meaningful clinical activity (12). Somatostatin receptors are expressed in approximately 90% of meningiomas, with previous case reports initially suggesting to target those receptors might have some therapeutic usefulness for patients with recurrent, unresectable meningiomas. Subsequent studies have not shown a clear benefit (13-16). Sunitinib, a tyrosine kinase inhibitor used to treat different solid cancers, showed modest activity in a small scale phase II trials that included grade 2 and 3 recurrent, pretreated meningioma (17).
Other promising actionable immunogenic biomarkers are the high-frequency microsatellite instability (MSI-H) and mismatch repair (MMR) deficiency. Those biomarkers serve as clinically validated predictors of response to immunotherapeutic agents such as pembrolizumab, which is currently FDA approved for all MSI-H solid tumors (18,19). One of the tumors to which NF-2 patients are at high risk of developing is meningiomas. They usually present as multiple intracranial or spinal meningiomas (20,21). They develop because sporadic meningiomas share the same genetic defect responsible for NF-2 disease. It is a mutation in the tumor suppressor gene NF2 located on chromosome 22q12 and encodes for a merlin protein. This merlin protein is responsible for linking the integral membrane proteins to the cytoskeleton of the cell. However, the mechanism of tumor formation is still unknown (21). Additional study has identified NF2 and MN1 (meningioma 1 gene) as candidate genes associated with malignant meningiomas (22). In contrast, the development of meningiomas in NF-1 is rare and not considered a characteristic finding of the disease (20,21). In addition, there is a lack of reporting of meningiomas in large population-based studies in NF1 patients and cohort studies of NF1 have so far not revealed any increase in the risk of meningiomas of any sort (23). There is a retrospective study in a pediatric age group suggesting that several risk factors can contribute to the development of meningiomas in children, and the absence of these factors did not exclude the meningioma risk; as sporadic meningiomas can also be seen in children. One of the most commonly observed risk factor for meningiomas in children in this study was NF. Eight out of 39 patients had a history of NF; four with NF-1 and four with NF-2. NF-2 has been reported in 6–39% of pediatric meningioma cases, as it has been shown to elevate the risk of developing intracranial meningiomas. NF-1 has not shown a similar increased incidence of meningioma development (24). However, in these four NF-1 patients, schwannomas, particularly cerebellopontine angle schwannomas have also been observed (24), although they are classical for NF-2 (25,26). It is also worth mentioning that the diagnosis of NF before its separation into NF-1 and NF-2 in 1987 is suspect, and the presence of schwannomas and particularly cerebellopontine angle schwannomas in these NF-1 patients suggests a massive doubt on the classification of NF in that study (27). Certain neoplasms are associated with NF-1, such as; rhabdomyosarcoma, carcinoid tumor, pheochromocytoma, and juvenile chronic myelogenous leukemia. However, sporadic and unrelated neoplasms such as malignant meningioma should be considered in the differential diagnosis of a mass in a patient with NF-1 (21). The study limitation was the lack of molecular testing of NF-1.
Conclusions
Grade 3 anaplastic meningioma is a rare tumor with a poor prognosis. To our knowledge, this is the first case report in English literature of grade 3 anaplastic meningioma in a patient with NF-1. Surgery followed by radiotherapy is recommended for the management of grade 3 meningiomas. However, no consensus on the management of recurrent meningiomas was established. The behavior of this tumor in our case was aggressive, similar to other cases of grade 3 meningiomas. TMZ was tried in this case following the failure of surgery and radiotherapy, and it did not show a good response. Systemic therapy in grade 3 meningiomas is still experimental and may have a slight clinical benefit. As a result, further prospective, multicentric studies are needed to ascertain these outcomes. Ultimately, discovering specific molecular biomarkers will allow us to suggest an individualized treatment. In fact, patients should be included in prospective trials because of the poor prognosis and aggressive nature of grade 3 meningiomas. This case suggests that the differential diagnosis of a mass in a patient with NF-1 should include tumors known to be associated with the syndrome as well as sporadic, unrelated neoplasms.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-8/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-8/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). We were not able to obtain written informed consent from the patient as she passed away a few years ago. An attempt was made to contact the patient’s family, but this was unsuccessful. Therefore, we followed our institution’s guidelines for case reports and reported the case after removing all personal health information, to ensure that the patient could not be identified by the history, figures, or pathology included in the report.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wilson TA, Huang L, Ramanathan D, et al. Review of Atypical and Anaplastic Meningiomas: Classification, Molecular Biology, and Management. Front Oncol 2020;10:565582. [Crossref] [PubMed]
- Farschtschi S, Mautner VF, McLean ACL, et al. The Neurofibromatoses. Dtsch Arztebl Int 2020;117:354-60. [PubMed]
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 2021;23:1231-51. [Crossref] [PubMed]
- Zhao L, Zhao W, Hou Y, et al. An Overview of Managements in Meningiomas. Front Oncol 2020;10:1523. [Crossref] [PubMed]
- Ogasawara C, Philbrick BD, Adamson DC. Meningioma: A Review of Epidemiology, Pathology, Diagnosis, Treatment, and Future Directions. Biomedicines 2021;9:319. [Crossref] [PubMed]
- Huntoon K, Toland AMS, Dahiya S. Meningioma: A Review of Clinicopathological and Molecular Aspects. Front Oncol 2020;10:579599. [Crossref] [PubMed]
- Smith MJ, Higgs JE, Bowers NL, et al. Cranial meningiomas in 411 neurofibromatosis type 2 (NF2) patients with proven gene mutations: clear positional effect of mutations, but absence of female severity effect on age at onset. J Med Genet 2011;48:261-5. [Crossref] [PubMed]
- Ozarslan B, Russo T, Argenziano G, et al. Cutaneous Findings in Neurofibromatosis Type 1. Cancers (Basel) 2021;13:463. [Crossref] [PubMed]
- Cao H, Jiang B, Zhao Y, et al. A rare subtype of meningioma: Case series of anaplastic meningioma and review of the literature. Medicine (Baltimore) 2018;97:e11019. [Crossref] [PubMed]
- Poniman J, Cangara MH, Kaelan C, et al. Progesterone Receptor Expression and Score Differences in Determining Grade and Subtype of Meningioma. J Neurosci Rural Pract 2020;11:552-7. [Crossref] [PubMed]
- Chamberlain MC, Tsao-Wei DD, Groshen S. Temozolomide for treatment-resistant recurrent meningioma. Neurology 2004;62:1210-2. [Crossref] [PubMed]
- Moazzam AA, Wagle N, Zada G. Recent developments in chemotherapy for meningiomas: a review. Neurosurg Focus 2013;35:E18. [Crossref] [PubMed]
- Hrachova M, Nguyen ENT, Fu BD, et al. A Retrospective Interventional Cohort Study to Assess the Safety and Efficacy of Sandostatin LAR for Treatment of Recurrent and/or Refractory Meningiomas. Front Neurol 2020;11:373. [Crossref] [PubMed]
- Wen PY, Quant E, Drappatz J, et al. Medical therapies for meningiomas. J Neurooncol 2010;99:365-78. [Crossref] [PubMed]
- Rünzi MW, Jaspers C, Windeck R, et al. Treatment of meningioma with octreotide. Lancet 1989;2:217-8. [Crossref] [PubMed]
- García-Luna PP, Relimpio F, Pumar A, et al. Clinical use of octreotide in unresectable meningiomas. A report of three cases. J Neurosurg Sci 1993;37:237-41. [PubMed]
- Kaley TJ, Wen P, Schiff D, et al. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol 2015;17:116-21. [Crossref] [PubMed]
- Dunn IF, Du Z, Touat M, et al. Mismatch repair deficiency in high-grade meningioma: a rare but recurrent event associated with dramatic immune activation and clinical response to PD-1 blockade. JCO Precis Oncol 2018; [Crossref] [PubMed]
- Marabelle A, Le DT, Ascierto PA, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol 2020;38:1-10. [Crossref] [PubMed]
- Goutagny S, Kalamarides M. Meningiomas and neurofibromatosis. J Neurooncol 2010;99:341-7. [Crossref] [PubMed]
- Pfeifer JD, Ashley Hill D, Ramos CV, et al. Meningioma presenting as an intraoral mass in a patient with neurofibromatosis type 1. Arch Pathol Lab Med 2000;124:898-901. [Crossref] [PubMed]
- Zhang X, Jia H, Lu Y, et al. Exome sequencing on malignant meningiomas identified mutations in neurofibromatosis type 2 (NF2) and meningioma 1 (MN1) genes. Discov Med 2014;18:301-11. [PubMed]
- McGaughran JM, Harris DI, Donnai D, et al. A clinical study of type 1 neurofibromatosis in north west England. J Med Genet 1999;36:197-203. [PubMed]
- Grossbach AJ, Mahaney KB, Menezes AH. Pediatric meningiomas: 65-year experience at a single institution. J Neurosurg Pediatr 2017;20:42-50. [Crossref] [PubMed]
- Hilton DA, Hanemann CO. Schwannomas and their pathogenesis. Brain Pathol 2014;24:205-20. [Crossref] [PubMed]
- Springborg JB, Poulsgaard L, Thomsen J. Nonvestibular schwannoma tumors in the cerebellopontine angle: a structured approach and management guidelines. Skull Base 2008;18:217-27. [Crossref] [PubMed]
- Ahn MS, Jackler RK, Lustig LR. The early history of the neurofibromatosis. Evolution of the concept of neurofibromatosis type 2. Arch Otolaryngol Head Neck Surg 1996;122:1240-9. [Crossref] [PubMed]
Cite this article as: AlAnsari GA, Bukhari N, Abdulkader MM, Alotain I, Taha MS. Malignant anaplastic meningioma in neurofibromatosis type 1 patient: a rare case report. AME Case Rep 2022;6:36.


