
Superficial solitary fibrous tumor masquerading as a dermoid cyst: a case report
Introduction
Solitary fibrous tumors (SFTs) are relatively rare mesenchymal neoplasms uncommonly encountered in dermatology practice. Originally described as a pleural tumor, SFTs in extra-pulmonary sites have become increasingly recognized (1,2). However, superficial SFTs (“cutaneous SFTs”) arising within the skin and soft tissue remain uncommon, with only case reports and limited case series noted in the literature to date (1). Periorbital superficial SFTs, in particular, are amongst the rarest presentations documented (1). Clinically, superficial SFTs manifest as solitary, unilateral, slow growing, non-descript superficial masses in patients above the age of 55 years, with symptoms primarily related to mass effect (1-3). Histologically, SFTs are composed of spindle cells arranged in a “patternless” growth pattern, with a rich hemangiompericytoma-like vascular component (also referred to as “staghorn” vessels) (1,4). The “molecular hallmark” of SFTs is the NGFI-A binding protein 2 (NAB2)-signal transducer and activator of transcription 6 (STAT6) fusion gene, present in the vast majority of cases (1,5,6). A high index of suspicion in the appropriate clinical context with special attention to clinicopathologic correlation is critical to accurate diagnosis of this rare entity.
We present the case of a 23-year-old woman with a slowly growing lateral supra-orbital mass, clinically concerning for a dermoid cyst, discovered to be a superficial SFT on pathologic examination. Her age and tumor location represent unique SFT manifestations in the literature, and STAT6 staining played a critical role in the diagnostic assessment of the lesion, further supporting the role of this immunohistochemical (IHC) stain in evaluation of these lesions. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-17/rc).
Case presentation
A 23-year-old, African American female presented to the dermatology clinic with concerns for a slowly enlarging nodule overlying the lateral aspect of her right eyebrow. The lesion had been present for many years prior to presentation and was causing her no discomfort. Desire for removal was based on cosmesis. Exam revealed a flesh-colored, soft, rubbery, mobile mass with no overlying skin changes, measuring 2.2 cm in diameter, overlying her right lateral supra-orbital rim (Figure 1).
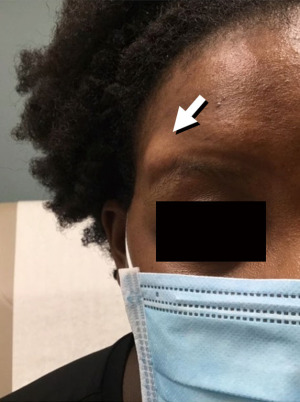
Due to clinical concern for an underlying dermoid cyst, imaging was performed to rule out connection to the central nervous system. Computed tomography showed a well-circumscribed subcutaneous nodule without connection to underlying structures; additionally, no pathologic regional lymph nodes were noted. Surgical excision was subsequently performed, and the nodule was sent for pathologic evaluation.
Histologic sections revealed a spindle cell neoplasm arranged in a “patternless” pattern, with hemangiopericytoma-like vessels dispersed throughout (Figure 2). The lesion stained diffusely positive for Bcl-2 and CD34, with patchy weak staining for NKIC3, CD68, and Factor XIIIa, as well as a low Ki-67 proliferation rate. Additional IHC staining for STAT6 was obtained and showed diffuse nuclear positivity, confirming the diagnosis of a superficial SFT (Figure 3). Risk-stratification classified the patient’s tumor as low risk for metastasis (Table 1) (7). Complete physical examination and review of systems was unremarkable at post-surgical follow-up and subsequent encounters, with no evidence of recurrence at one year follow-up.
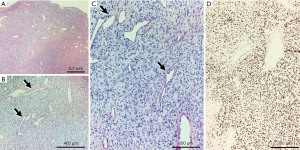
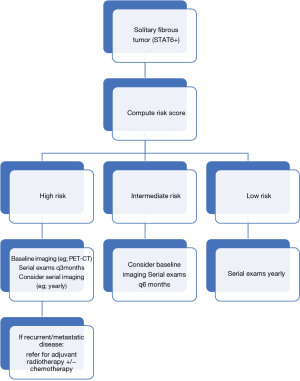
Table 1
| Risk factor | Score | Present case |
|---|---|---|
| Age (years) | ||
| <55 | 0 | 0 |
| ≥55 | 1 | |
| Tumor size (cm) | ||
| <5 | 0 | 0 |
| [5–10) | 1 | |
| [10–15) | 2 | |
| ≥15 | 3 | |
| Mitotic count (mitoses/10 high-power fields) | ||
| 0 | 0 | 0 |
| 1–3 | 1 | |
| ≥4 | 2 | |
| Tumor necrosis | ||
| <10% | 0 | 0 |
| ≥10% | 1 | |
| Risk class | Total score | |
| Low | 0–3 | 0 |
| Intermediate | 4–5 | |
| High | 6–7 | |
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
SFTs are uncommon spindle cell neoplasms of fibroblastic/myofibroblastic lineage originally described as arising within the pleural cavity (1,2). Extra-pleural SFTs have been subsequently reported in various other locations, including in the skin and its immediate subcutis, where they are termed superficial SFTs (1,8). Case series suggest that superficial SFTs most commonly present on the head and neck of middle-aged females (1). Typically, they are removed for cosmetic reasons, often being mistaken clinically for a benign adnexal neoplasm or cyst, as was the case in our patient. Radiological imaging is generally non-specific, showing a solid nodular mass with well circumscribed borders—making the diagnosis of SFT largely one based on histology (2). Our case aligns with previous reports of superficial SFTs in several aspects including female gender, head/neck location, non-specific imaging findings, and slow growth over several years; however, several aspects are unique. Our patient reported onset in childhood and presented with a lateral supraorbital location, a presentation that may lead to the misdiagnosis of a dermoid cyst. A distinguishing feature that can be elicited by history would be the exact time of onset, with majority of dermoid cysts apparent in early childhood, whereas our case presented in later childhood.
On histologic analysis SFTs are primarily comprised of spindled cells in a “patternless” pattern admixed within a variable amount of thick keloidal collagen and prominent hemangiopericytoma-like vessels (1,8). The differential diagnosis of spindle cell neoplasms is broad, including both benign and malignant entities, and has been previously reviewed (1,6,9). Differentiating superficial SFTs from more aggressive spindle cell neoplasms (e.g., dermatofibrosarcoma protuberans, malignant peripheral nerve sheath tumor) may be challenging and IHC staining is often required for a definitive diagnosis. Historically, the combination of typical morphology with IHC staining for CD34, CD99, and Bcl-2 was required for the diagnosis of SFT (6). Limitations to specificity were acknowledged with this diagnostic profile, though, and the recent discovery that SFTs uniformly possess a NAB2-STAT6 fusion gene which correlates with STAT6 IHC staining has revolutionized the diagnostic assessment of these tumors—especially in clinical settings where this entity is unexpected and other spindle cell tumors may be more common (2,4-6,10). Due to these findings, we suggest that STAT6 be added to the panel of immunoperoxidase stains utilized by pathologists when confronted with a spindle cell neoplasm of unknown lineage.
Due to the low incidence of disease, there is limited data to guide prognostic discussions and treatment recommendations for these tumors. Considered tumors of intermediate biologic potential, SFTs have shown the potential to recur and metastasize (1). As such, complete resection is generally recommended (1,3). Histology is a helpful but imperfect method of predicting biologic behavior, with increasing tumor size, patient age, mitotic rate, and presence of necrosis correlating with high-risk tumors (Table 1) (7,10). Location of the tumor may also play a role in predicting behavior of SFTs (e.g., superficial versus deep soft tissue). The largest case series to date evaluating specifically superficial SFTs, found no evidence of recurrence or metastasis in all cases, with a median follow-up time of 16 months (1). This suggests that superficial SFTs behave more indolently compared to their deeper counterparts; whether this is due to easier accessibility (e.g., for tumor extirpation prior to sustained growth) or other factors remains to be determined. Long term follow-up after complete excision is critical to monitor for recurrence and/or metastasis; a proposed approach to management of superficial SFTs is included (Figure 3). Importantly, our case showed no high-risk features and has not recurred nor metastasized at one year follow-up, further validating the risk-stratification model proposed by Demicco et al. (7).
In conclusion, we present the case of a young adult female with a slow growing supraorbital superficial SFT for which recognition of key histopathologic features and STAT6 staining played a critical role in diagnosis. The young age of onset and lateral supraorbital location were atypical for SFT leading to an initial misdiagnosis of dermoid cyst, confirming the importance of histologic analysis for these lesions. Understanding that this uncommon tumor can present in skin and soft tissues and has a unique IHC pattern is important for accurate and timely diagnosis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-17/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-17/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Feasel P, Al-Ibraheemi A, Fritchie K, et al. Superficial Solitary Fibrous Tumor: A Series of 26 Cases. Am J Surg Pathol 2018;42:778-85. [Crossref] [PubMed]
- Smith SC, Gooding WE, Elkins M, et al. Solitary Fibrous Tumors of the Head and Neck: A Multi-Institutional Clinicopathologic Study. Am J Surg Pathol 2017;41:1642-56. [Crossref] [PubMed]
- Shen J, Li H, Feng S, et al. Orbital solitary fibrous tumor: a clinicopathologic study from a Chinese tertiary hospital with a literature review. Cancer Manag Res 2018;10:1069-78. [Crossref] [PubMed]
- Magro G, Angelico G, Leone G, et al. Solitary fibrous tumor of the breast: report of a case with emphasis on diagnostic role of STAT6 immunostaining. Pathol Res Pract 2016;212:463-7. [Crossref] [PubMed]
- Robinson DR, Wu YM, Kalyana-Sundaram S, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet 2013;45:180-5. [Crossref] [PubMed]
- Doyle LA, Vivero M, Fletcher CD, et al. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol 2014;27:390-5. [Crossref] [PubMed]
- Demicco EG, Wagner MJ, Maki RG, et al. Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Mod Pathol 2017;30:1433-42. [Crossref] [PubMed]
- Kiyohara T, Tanimura H, Takewaki H, et al. Malignant solitary fibrous tumor in the subcutis: Report of a rare superficial malignant type and review of published work. J Dermatol 2019;46:267-70. [Crossref] [PubMed]
- Tariq MU, Din NU, Abdul-Ghafar J, et al. The many faces of solitary fibrous tumor; diversity of histological features, differential diagnosis and role of molecular studies and surrogate markers in avoiding misdiagnosis and predicting the behavior. Diagn Pathol 2021;16:32. [Crossref] [PubMed]
- Machado I, Nieto Morales MG, Cruz J, et al. Solitary Fibrous Tumor: Integration of Clinical, Morphologic, Immunohistochemical and Molecular Findings in Risk Stratification and Classification May Better Predict Patient outcome. Int J Mol Sci 2021;22:9423. [Crossref] [PubMed]
Cite this article as: Foss MG, Dunn C, Marks E, Nathoo R. Superficial solitary fibrous tumor masquerading as a dermoid cyst: a case report. AME Case Rep 2022;6:34.


