The unusual first sign of presentation of renal cell carcinoma: a rare case report
Introduction
Kidney cancer is estimated to account for approximately 5% of new cancer diagnoses in men and 3% of new cancer diagnoses in women in the US in 2020 (1). Renal cell carcinoma (RCC) accounts for approximately 90% of kidney cancers, while clear cell, papillary and chromophobe subtypes account for approximately 90% of renal cell cancers (2). The peak of RCC incidence is in the sixth decade with nearly twice incidence in men as compared with women. The incidental detection of RCC is about 50% of cases; in fact, nowadays, the classic triad of presenting symptoms for renal cancer (hematuria, flank pain, and palpable mass) is a rare finding, usually observed in case of advanced disease (3). At the time of diagnosis, about 54% of patients present organ-confined disease, 20% locally advanced disease, and the remaining 25% have metastatic disease (4). The most common sites of metastases are lung (45%), lymph nodes (22%), bones (30%), and liver (20%) (5). RCC ranks as the third neoplasm among the malignancies that metastasize most commonly to anatomic sites of head and neck, after breast and lung cancer (6). Orbital and intraocular metastases of RCC are rare, accounting together for less than 2% of all ophthalmic metastasis. In individual series, the eye is involved in 2% to 5% of cases of metastatic disease, whereas the orbit is involved in 1% to 5%, representing sometimes the first clinical manifestation of kidney cancer (7).
This article aimed to present a patient with ptosis and exophthalmos, due to orbital metastasis, as the first manifestation of a previously undetected RCC. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-16/rc).
Case presentation
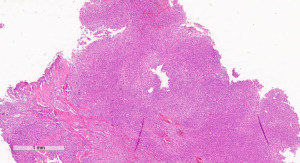
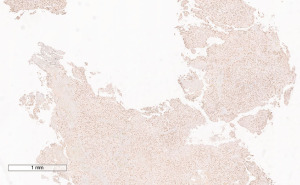
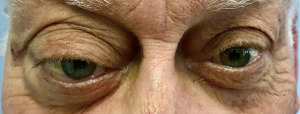
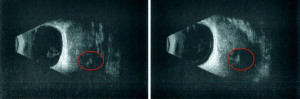
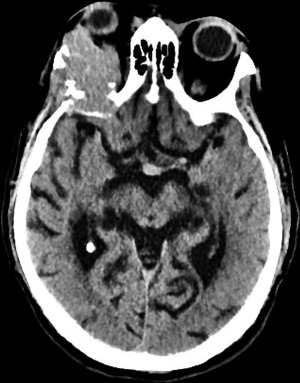
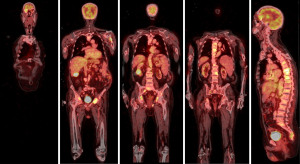
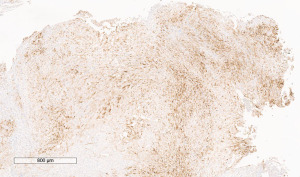
A 72-year-old man, presented to our ophthalmology department reporting a mass in the right eye accompanied by throbbing pain and vision loss. Past medical history was notable for type II diabetes mellitus, gouty tophus, essential hypertension, chronic kidney disease, a third-degree atrioventricular block that required a pacemaker implant. He had neither personal nor family history of cancer. The physical examination revealed third-grade exophthalmos on the right eye and a firm and fixed mass on the right superior orbit on palpation (Figure 1). Palpebral ptosis and conjunctival hyperemia, with vision loss and slightly limited right eye movements, were also present. Ultrasonography of the right eye showed the presence of a large mass arising from the lacrimal gland, extending to the temporal lobe and occupying I/II/III Benedikt’s space, compatible with lymphoma (Figure 2). Cranial computed tomography scan (CT scan) with contrast demonstrated the presence of an expansive lesion, with a maximum diameter of 50×30 mm on axial plane, located at the upper outer portion of the right orbit, right anterior clinoid process, and right lesser wing of the sphenoid bone. The lesion infiltrated the right lateral rectus muscle and part of the right superior rectus muscle and caused the medial displacement of the right optic nerve, with initial signs of pachymeningeal enhancement in the right front-orbital area (Figure 3). Tumor mass showed irregular contrast enhancement. To obtain tumor tissue for pathology assessment, the patient underwent an orbitotomy. A subsequent 18-fluorodeoxyglucose-positron emission tomography/computerized tomography scan (18F-FDG PET/CT scan) showed a lesion on the right orbit measuring approximately 25×38 mm, with diffuse tracer uptake in the right orbit (SUVmax 5.5), lateral and posterior wall erosion of orbit and with extra-orbital extension, subcentimetric multiple lung nodules, the greater located on inferior lobe (SUVmax 3.9), a hyperdense lesion enclosing hypodense necrotic areas in the lower third of right kidney with a maximum diameter of 50 mm (SUVmax 12.3), and disseminated bone involvement with mixed lytic and sclerotic bone metastases (Figure 4). We finally got the histological examination that proved the presence of a malignant lesion, likely epithelial, highly suggestive of a secondary lesion from renal cell carcinoma. Immunohistochemistry showed positivity for paired-box gene 8 (PAX8), cytokeratin 19 (CK 19) and renal cell carcinoma marker (RCC-Ma) and negativity for S100, thyroid transcription factor-1 (TTF1), human melanoma black-45 (HMB45), p63, cytokeratin 7 (CK 7), placental alkaline phosphatase (PLAP) and GATA binding protein 3 (GATA 3) (Figures 5-7). Therefore, the patient underwent palliative radiation therapy delivered to the orbital lesion with the scope to relieve pain, with a total dose of 20 Gy in 5 fractions. He subsequently started systemic therapy with pazopanib, a multi-target tyrosine kinase inhibitor (TKI) used routinely in the treatment of advanced renal cancer, at the dose of 800 mg daily. Unfortunately, he did not achieve any benefit from systemic therapy, his conditions progressively worsened, and he finally passed away after four months of treatment due to rapid progression of disease. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for the publication of this case report, including radiographic images and patient picture was obtained from the patient’s daughter. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Overall, metastatic dissemination to the orbit is uncommon in cancer patients; indeed, only 2–5% of them will develop a secondary lesion in this anatomic site (8,9). Moreover, about only 1% to 13% of orbital mass lesions are represented by metastases. Secondary orbital lesions, as reported in clinical cases, are typically found in advanced cancer patients with an age between 51 and 60 years old and occur 19 months to 7 years after the primary tumor diagnosis (8). The most frequent malignancies spreading to the orbit are breast, lung, and prostate cancers, as well as melanoma and skin cancers. In more than 25% of cases, orbital metastases are the first manifestation of a primary tumor of unknown origin (10). Generally, orbital metastases can occur with 5 different clinical patterns: mass effect, infiltrative tumor growth pattern, functional impact, signs of inflammation or no symptoms (11). Some of these clinical manifestations can coexist. Kidney cancer is usually a well-encapsulated tumor, with a clinical behavior characterized by slow growth, although it often presents an unpredictable evolution and displays metastatic potential (12). The most common sites of kidney cancer metastases are lung (45%), lymph nodes (22%), bone (30%), and liver (20%) (5). Metastases to the head and neck region have been found in 15% of cases, most frequently involving the nose, paranasal sinuses, and oral cavity. Ocular metastases from kidney cancer usually involve the iris, ciliary body, and choroids, although eyelid and lacrimal sac involvement have also been described (13). Therefore, orbital metastases from kidney cancer are very rare.
The clinical features of orbital metastases from kidney cancer are non-specific and could divert attention from the real problem. Indeed, ophthalmologic symptoms, like ptosis, diplopia, vision loss, epiphora, strabismus, and cataract, could rely on many factors, such as size, type, and location of metastasis and are not suggestive of the nature and origin of the primary tumor (6). Hence, in the absence of pathognomonic symptoms of kidney cancer, imaging is crucial. A total body CT scan is necessary to orient the diagnosis, rule out other secondary lesions and identify the ideal site for tissue biopsy. In this case, the orbital biopsy was essential to identify the malignant nature of the lesion, as well as its histological features.
The mechanism by which kidney cancer metastasizes to the orbit is still unknown. One potential mechanism of tumor diffusion to the orbital area is a hematogenous spread of circulating tumor cells, as the eye has no lymphatic channels. Thereby, tumor cells reach the orbit by entering the circulatory system and spreading to the lung first. Tumor cells migration with retrograde venous flow through the vertebral-basilar plexus is another potential mechanism (14).
During the last decades, many advances have been made for the treatment of metastatic RCC. The drastic change happened when the new combinations of immune checkpoint inhibitors (ICIs) with tyrosine kinase inhibitors (TKIs) or the association of anti-PD-1 plus anti CTLA-4 improved the therapeutic portfolio, especially for patients with intermediate and poor risk class.
Indeed, as emerged from the Keynote 426 study, the combination of Pembrolizumab plus Axitinib, or from the CLEAR and Checkmate ER trials, the combination of Pembrolizumab plus Lenvatinib and Nivolumab plus Cabozantinib, respectively, demonstrated to be more effective as compared to sunitinib monotherapy. At the same time, the combination of Nivolumab and Ipilimumab showed significantly superior overall survival (OS) and overall response rate (ORR) compared to sunitinib for intermediate and poor risk patients (Table 1).
Table 1
| Study | Primary endpoint | Experimental arm | OS (mo) HR | PFS (mo) HR |
|---|---|---|---|---|
| KEYNOTE 426 | PFS and OS in the ITT | Pembrolizumab plus axitinib vs. sunitinib | 45.7 vs. 40.1; HR: 0.73 (0.60–0.88) | 15.7 vs. 11.1, HR: 0.68 (0.58–0.80) |
| Checkmate 214 | PFS and OS in the IMDC intermediate and poor population | Nivolumab plus ipilimumab for 4 doses then nivolumab vs. sunitinib | ITT: 55.7 vs. 38.4; HR: 0.72 (0.62–0.85) | ITT: 12.3 vs. 12.3; HR: 0.86 (0.73–1.01) |
| I/P: 47 vs. 26.6; HR: 0.68 (0.58–0.81) | I/P: 11.6 vs. 8.3; HR: 0.73 (0.61–0.87) | |||
| CheckMate 9ER | PFS in the ITT | Nivolumab plus cabozantinib vs. sunitinib | NR vs. NR; HR: 0.60 (0.40–0.89) | 16.6 vs. 8.3; HR: 0.51 (0.41–0.64) |
| CLEAR | PFS in the ITT | Pembrolizumab plus lenvatinib vs. sunitinib | NR vs. NR; HR: 0.66 (0.49–0.88) | 23.9 vs. 9.2 HR: 0.39 (0.32–0.49) |
| Everolimus plus lenvatinib vs. sunitinib | NR vs. NR; HR: 1.15 (0.88–1.50) | 14.7 vs. 9.2; HR: 0.65 (0.53–0.80) | ||
| Javelin Renal 101 | OS in the ITT and PFS in the PD-L1+ population | Avelumab plus axitinib, vs. sunitinib | ITT: NE vs. 37.8; HR: 0.67 (0.57–0.79) | ITT: 13.9 vs. 8.5; HR: 0.69 (0.56–0.84) |
| PD-L1: NE vs. 29.6; HR: 0.83 (0.50–1.15) | PD-L1: 13.8 vs. 7; HR: 0.62 (0.49–0.77) |
PFS, progression-free survival; OS, overall survival; ITT, intention-to-treat; IMDC, International mRCC Database Consortium; HR, hazard ratio; mo, months; NR, not reached; I/P, intermediate/poor risk; NE, could not be estimated.
Despite all the recent progress in anticancer therapy, patient prognosis is still poor in the presence of orbital metastases, maybe due to the high systemic cancer burden and/or to a particularly aggressive disease. Indeed, there are currently no available data demonstrating a clear clinical benefit achieved by combination therapies in the management of patients with orbital metastases from kidney cancer. However, the combination of TKI plus anti PD-1 might be the preferred option when a rapid tumor response is needed due to disease burden and related symptoms, given considering the reported response rates, which were greater than those obtained with ipilimumab plus nivolumab (15,16).
A recent review showed a median OS of 6 months (range, 0.2–144 months), and a 2-year survival rate of 29% for all cases of cancer patients with orbital metastases (17). Long-term outcome has not significantly improved over time and systemic and locoregional treatments are mostly palliative (8).
Despite the diagnostic delay, due to the unusual clinical presentation, this case suggests a poor efficacy of TKI monotherapy, as patient survival was only 4 months from treatment start.
The radiotherapy treatment resulted in the greatest clinical benefit achieving a pain reduction.
In summary, we described here a rare case of orbital metastasis, as the first and unique sign of a previously undetected RCC. Since ophthalmic signs and symptoms are not pathognomonic of any cancer type, their occurrence can suggest different clinical conditions resulting in delayed diagnosis and treatment. Therefore, despite its rarity, differential diagnosis of an orbital lesion should always consider the possibility of metastasis from kidney cancer.
Acknowledgments
This report was part of research activity of the Rare Tumors Coordinating Center of Campania Region (CRCTR) recognized as full member of the European Reference Network (ERN-EURACAN). The authors would like to acknowledge the ERN-EURACAN as a powerful resource for transnational collaboration in rare cancers.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-16/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-16/coif). SDP reports consulting fees for Consulting or advisory Role: GSK, MSD, Seagen, Daiichi Sankyo, Lilly, Clovis, Celgene, Astrazeneca, Novartis, Pfizer, Roche; and Speaker’s Bureau: Celgene, Astrazeneca, Novartis, Pfizer, Roche. MG reports consulting fees for Consulting or advisory Role: Astrazeneca, MSD, Seagen, Daiichi Sankyo, Lilly, Celgene, Novartis, Pfizer; Speaker’s Bureau: Lilly, Celgene, Novartis, Pfizer, Istituto Gentili, Eisai Europe Ltd., Roche; Travel, accommodation, expenses: Novartis, Pfizer, Roche. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for the publication of this case report, including radiographic images and patient picture was obtained from the patient’s daughter. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Inamura K. Renal Cell Tumors: Understanding Their Molecular Pathological Epidemiology and the 2016 WHO Classification. Int J Mol Sci 2017;18:2195. [Crossref] [PubMed]
- Lázaro M, Valderrama BP, Suárez C, et al. SEOM clinical guideline for treatment of kidney cancer (2019). Clin Transl Oncol 2020;22:256-69. [Crossref] [PubMed]
- Heldwein FL, McCullough TC, Souto CA, et al. Localized renal cell carcinoma management: an update. Int Braz J Urol 2008;34:676-89; discussion 689-90. [Crossref] [PubMed]
- Gong J, Maia MC, Dizman N, et al. Metastasis in renal cell carcinoma: Biology and implications for therapy. Asian J Urol 2016;3:286-92. [Crossref] [PubMed]
- Sountoulides P, Metaxa L, Cindolo L. Atypical presentations and rare metastatic sites of renal cell carcinoma: a review of case reports. J Med Case Rep 2011;5:429. [Crossref] [PubMed]
- Rai R, Jakobiec FA, Fay A. Ocular Metastatic Renal Carcinoma Presenting With Proptosis. Ophthalmic Plast Reconstr Surg 2015;31:e100-8. [Crossref] [PubMed]
- Wladis EJ, Lee KW, Nazeer T. Metastases of systemic malignancies to the orbit: a major review. Orbit 2021;40:93-7. [Crossref] [PubMed]
- Magliozzi P, Strianese D, Bonavolontà P, et al. Orbital metastases in Italy. Int J Ophthalmol 2015;8:1018-23. [PubMed]
- Ahmad SM, Esmaeli B. Metastatic tumors of the orbit and ocular adnexa. Curr Opin Ophthalmol 2007;18:405-13. [Crossref] [PubMed]
- Goldberg RA, Rootman J, Cline RA. Tumors metastatic to the orbit: a changing picture. Surv Ophthalmol 1990;35:1-24. [Crossref] [PubMed]
- Lieder A, Guenzel T, Lebentrau S, et al. Diagnostic relevance of metastatic renal cell carcinoma in the head and neck: An evaluation of 22 cases in 671 patients. Int Braz J Urol 2017;43:202-8. [Crossref] [PubMed]
- Shome D, Honavar SG, Gupta P, et al. Metastasis to the eye and orbit from renal cell carcinoma--a report of three cases and review of literature. Surv Ophthalmol 2007;52:213-23. [Crossref] [PubMed]
- Croxatto JO. Mechanisms of Tumor Metastasis in the Orbit. In: Orbital Tumors. New York, NY: Springer, 2005;29-36.
- Fitzgerald KN, Lee CH. Personalizing First-Line Management of Metastatic Renal Cell Carcinoma: Leveraging Current and Novel Therapeutic Options. J Natl Compr Canc Netw 2022; Epub ahead of print. [Crossref] [PubMed]
- Govindarajan A, Castro DV, Zengin ZB, et al. Front-Line Therapy for Metastatic Renal Cell Carcinoma: A Perspective on the Current Algorithm and Future Directions. Cancers (Basel) 2022;14:2049. [Crossref] [PubMed]
- Palmisciano P, Ferini G, Ogasawara C, et al. Orbital Metastases: A Systematic Review of Clinical Characteristics, Management Strategies, and Treatment Outcomes. Cancers (Basel) 2021;14:94. [Crossref] [PubMed]
Cite this article as: Morra R, D’Ambrosio A, Pietroluongo E, De Placido P, Montella L, Del Deo V, Tortora M, Matano E, Damiano V, Palmieri G, De Placido S, Giuliano M. The unusual first sign of presentation of renal cell carcinoma: a rare case report. AME Case Rep 2022;6:35.