
Delayed complication of iliac artery aneurysm repair—from critical limb ischemia to mixed shock: a case report
Introduction
Iliac artery aneurysms (IAA) are permanent, focal dilatation of the iliac artery with minimum 50% increase in diameter compared to normal artery (1). They are more common in men, with incidence and size of aneurysm increasing with age. Although often asymptomatic, they can cause local compression of pelvic structures (sacral plexus, colon, ureter, iliac vein), thrombosis, embolism or rupture (2,3). While endovascular repair has become the preferred method in recent years, for patients with extensive aneurysmal disease, the standard of care is surgical procedure (4).
Acute limb ischemia (ALI) related to aneurysmal disease can occur by progression of untreated disease or rarely, as a complication of its repair. Its management requires early identification and prompt revascularization.
We present a case of a patient with prior aorto-bifemoral bypass graft repair of bilateral aortoiliac aneurysmatic disease who presented nine years later with critical limb ischemia due to graft compression by a massively enlarged post-repair left IAA. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-9/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Despite multiple attempts at reaching the patient’s family for written informed consent, we were unable to establish contact with them. Therefore, publication of this case report and accompanying images was obtained from institutional exception as the manuscript contains no patient identifiers and is fully anonymous. The manuscript is therefore compliant with all relevant Health Insurance Portability and Accountability Act (HIPAA), common rule and institutional regulations.
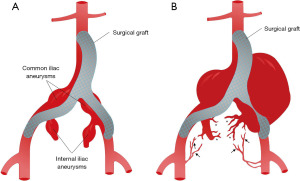
A man in his 70s with heart failure, hypertension, end stage renal disease on hemodialysis and severe peripheral vascular disease, who had undergone aorto-bifemoral repair (bifurcated Hemashield graft, Maquet®, Germany) for infra-renal abdominal aortic aneurysm (3.6 cm), bilateral common (3.3 cm left, 3.6 cm right) and bilateral internal iliac aneurysms (3.7 cm left, 3.0 cm right) nine years prior (Figure 1), presented to the emergency department (ED) with 3 weeks of severe and progressively worsening left leg pain.
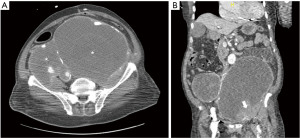
His physical exam was remarkable for distended abdomen and loss of left popliteal, tibial, and dorsalis pedis pulses with a cold below-the-knee left lower extremity and limited motor function. Arterial ultrasound of the lower extremity showed occlusion of left common femoral, profunda, popliteal and anterior tibialis arteries. Computed tomography (CT) of the abdomen and pelvis with contrast (Figure 2A,2B) showed large cystic lesions within the pelvis encasing the aorta related to aneurysm dilatations of the iliac arteries. A thrombus within the left common femoral artery and contrast extravasation within the dilated left iliac aneurysm sac were seen.
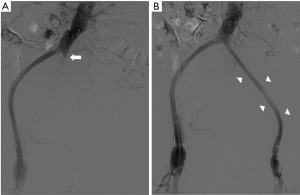
Emergent aortogram revealed left iliac graft occlusion and stenosis of the left graft limb femoral anastomosis, as well as late retrograde collateral blood flow to the aneurysm coming from trans pelvic collaterals and branches of left profunda femoris artery (Figure 1B). He underwent left femoral artery thrombectomy, bilateral graft limb stent placement and left femoral graft anastomosis balloon angioplasty and stent placement with restoration of blood flow to the left lower extremity (Figure 3). Several days after reperfusion of the limb, the patient distended and tense abdomen rose concerns for the development of compartment syndrome. This was attributed to the large hematoma in the abdominal cavity from the IAA expansion. He underwent ultrasound guided catheter evacuation of two liter of blood from the left aneurysm sac in efforts to palliate symptoms. Follow up CT angiography revealed no active aneurysmatic leak with adequate hemostasis. There was no follow up CT abdomen done after percutaneous drainage as clinical improvement were based on physical exam and patient’s condition. Upon discharge, he was instructed to return as outpatient in one month but was lost in follow up.
Eight months later, the patient returned to the ED with lower abdominal pain radiating to the back and non-bloody diarrhea for one day. CT of abdomen and pelvis revealed bilateral expanding contiguous pelvic aneurysms composed of distal abdominal aortic aneurysm, bilateral common iliac aneurysms, bilateral internal and external iliac aneurysms that occupied almost half of the abdominal cavity measuring up to 23 cm on the left side with associated moderate hemoperitoneum concerning for rupture. He underwent emergent coil embolization of multiple vascular branches of the left internal iliac artery and cyanoacrylate glue embolization of a single collateral feeder to the leak in efforts to achieve hemostasis. Ongoing hemorrhagic shock from massive hemoperitoneum and obstructive shock from the onset of abdominal compartment syndrome (ACS) required initial stabilization in the intensive care unit (ICU) followed by transportation to the operating room. He had a high mortality risk and efforts to address the aneurysm were limited due to its large size and complex anatomy. Instead, efforts focused on palliation of ACS and abdominal decompression.
Upon opening of the abdominal fascia, a very tense aneurysmatic sac was seen right next to the abdominal wall which was accessed and large amounts of blood and aneurysmatic clot evacuated as fresh bleeding was seen coming from underneath the aneurysmal sac. Refractory intra-operatory hypotension with massive transfusion protocol activation precluded further exploration for all aneurysmal sac features. Abdominal packing was performed, fascia approximated, and skin closed. Patient was transferred back to the ICU for further stabilization. Two days later, he returned to the operating room. Remaining hemoperitoneum was drained as well as debris from the aneurysmatic sac. There was visualization of major bleeding from the inferior aspect of the left iliac aneurysmatic sac related to retrograde flow from the femoral artery which resolved with approximation of the aneurysmal wall. Abdomen was closed and patient was transferred back to the ICU. Despite aggressive post-operative care, requiring maximal vasopressors support, refractory multiorgan failure persisted and progressed leading to pulses electrical activity and patient’s demise.
Discussion
Our case illustrates the clinical course of a patient with prior bilateral aorto-common iliac-internal iliac aneurysmal disease status post surgical bypass aorto-femoral graft repair who developed critical limb ischemia due to extrinsic compression of vascular graft with vascular stasis and thrombus formation due to the progression of his aneurysmatic disease over the course of eight years. This case highlights an infrequent etiology of ALI and further describes the clinical evolution of a patient with progressive severe IAA disease despite endovascular and surgical interventions.
Iliac aneurysms are a rare phenomenon compromising less 2% of all abdominal aneurysms. Spontaneous rupture is a complication of abdominal aneurysms that carries mortality rates as high as 33% for endovascular and 50% for surgical repair (5). The decision to treat via open surgical or endovascular repair depends on factors such as anatomy of aneurysm including diameter, tortuosity and angulation of vessels, extent of aneurysmal disease, patient comorbidities, and urgency of repair (6,7). Although the decision for surgical intervention and characteristic of the initial IAA were not available to the authors, we assumed that a surgical approach was chosen due to its complex vascular features at that time.
More than 66% of patients presenting with ruptured aneurysm sac had insufficient or no routine surveillance (8). Physical exam can be unrevealing, with most guidelines placing an emphasis on routine imaging such as ultrasound or CT angiogram to screen for recurrence or complications (9,10). Lifelong surveillance must be addressed clearly in clinical guidelines, as complications have been reported for both interventions endovascular aneurysm repair (EVAR) and open, up to 15 years after (11-13). In our case, the complication occurred 8 years later and proper follow up might have changed his fatal outcome. We infer that our patient, and in accordance to prior reported cases, had an incomplete vascular obliteration or lack of internal iliac artery ligation during initial surgical repair allowing for the development of collateral vessels and retrograde flow into the aneurysm over the course of several years (3,14). Over time, extrinsic compression led to vascular stasis and eventual femoral artery thrombus formation directing our patient to seek emergent medical attention for life-threatened limb. However as stated previously, we lack the operative details to confirm any details regarding the initial surgical repair. In regards to the initial surgery it was surgeon preference to perform aorto-bifemoral bypass as opposed to aorto-biiliac graft during initial surgery. We can infer that the purpose was to exclude/bypass the iliac arterial system (common and internal iliac artery) from systemic circulation since this comprised the severe aneurysmal disease. Based on interventions done during hospitalization related in this paper, we can also deduce that the internal iliac artery was not ligated (either properly or at all) and only the external iliac artery was ligated.
While typically open surgical repair may not require close surveillance, we suggest that in open surgical repairs where surgical details cannot be fully confirmed, surveillance imaging should be performed for early detection of untreated or progression of initial disease.
The ligation of the internal iliac artery to prevent retrograde filling of the aneurysm could result in pelvic ischemia presenting as buttock claudication or necrosis, impotence and/or left colon ischemia (15). Perhaps preventing pelvic ischemia can explain why the original surgeons did not ligate the internal iliac artery. Rarely, acute lower limb ischemia can result from graft thrombosis or distal embolization during surgical manipulation (16). Our patient’ initial presentation of ALI was unique as it was rather driven by decreased blood flow, vascular stasis and thrombus formation from graft extrinsic compression due to a massively dilated iliac aneurysm that had progressed over several years.
Despite initial endovascular stabilization and plans for close follow up after his initial hospital admission, our patient returned to the hospital with a ruptured of his complex IAA and had developed ACS and obstructive shock. ACS is defined as pressure in the abdominal cavity above 20 mmHg with associated organ dysfunction. The etiology of our patient’s compartment syndrome was multifactorial as the presence of hemoperitoneum, the mass effected caused by massive IAA and the low intravascular preload state due to active hemorrhage, all contributed to its presentation and severity. In addition to the obstructive component from ACS, a hypovolemic component from active arterial bleed was presented in our patient consistent with a clinical picture of mixed shock. The multifactorial management of mixed shock is target to the synchronous etiologies. Our patient required immediate release of the obstruction by decompressive laparotomy and aggressive preload repletion by means of massive transfusion guidelines. Mortality from ACS related to abdominal aneurysm rupture is close to 50% and nearly always fatal if ACS, of any cause, is left untreated (17).
In complex cases, such the one presented, the current grade 1D recommendation is decompressive laparotomy with temporary abdominal closure as needed (18). Surgical decompression decreases mortality rates and improves outcomes (17,19). Even after decompressive surgery, mortality rates remains as high as 50% and the longer the abdomen is open, the greater potential for morbidity (20).
Our case illustrates an unusual mechanism of ALI by the gradual expansion of the original left-sided IAA over the course of eight years by late retrograde collateral flow repressurizing and filling the original aneurysms from trans pelvic collaterals and branches of left profunda femoris artery that eventually reach a size able to compress extrinsically and occlude the left graft limb completely. Furthermore, the natural progression of his disease featuring life-threatening conditions is shared with the reader with the onset of obstructive shock from ACS and hemorrhagic shock from arterial extravasation as major driver for the development of multiorgan failure leading to our patient’s cardiac arrest and death.
To our review, this is the first reported case of initial ALI secondary to compression of a bypass graft by a re-expanding IAA years after surgical repair with eventual rupture, with the eventual development of mixed shock and dead despite endovascular and surgical interventions. Although rare in the literature, IAA should warrant close attention and prolonged follow up by clinicians, and high index of suspicion and consideration of its potential complications after surgical repair.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-9/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-22-9/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-9/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Despite multiple attempts at reaching the patient’s family for written informed consent, we were unable to establish contact with them. Therefore, publication of this case report and accompanying images was obtained from institutional exception as the manuscript contains no patient identifiers and is fully anonymous. The manuscript is therefore compliant with all relevant HIPAA, common rule and institutional regulations.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bacharach JM, Slovut DP. State of the art: management of iliac artery aneurysmal disease. Catheter Cardiovasc Interv 2008;71:708-14. [Crossref] [PubMed]
- Thompson PM, Packham DA, Yates-Bell AJ. Ureteric obstruction of solitary kidneys by aneurysms of the iliac artery. Br J Urol 1981;53:421-3. [Crossref] [PubMed]
- Soury P, Brisset D, Gigou F, et al. Aneurysms of the internal iliac artery: management strategy. Ann Vasc Surg 2001;15:321-5. [Crossref] [PubMed]
- Nakajima T, Kawazoe K, Komoda K, et al. Failure of exclusion of internal iliac artery aneurysms. J Vasc Surg 2001;33:476-80. [Crossref] [PubMed]
- Chaer RA, Barbato JE, Lin SC, et al. Isolated iliac artery aneurysms: a contemporary comparison of endovascular and open repair. J Vasc Surg 2008;47:708-13. [Crossref] [PubMed]
- Krajcer Z. Treating Iliac Aneurysms. Endovasc Today 2005;May:48-51. Available online: http://evtoday.com/pdfs/EVT0505_F6_Krajcer.pdf
- Taudorf M, Jensen LP, Vogt KC, et al. Endograft limb occlusion in EVAR: iliac tortuosity quantified by three different indices on the basis of preoperative CTA. Eur J Vasc Endovasc Surg 2014;48:527-33. [Crossref] [PubMed]
- Antonopoulos CN, Kakisis JD, Giannakopoulos TG, et al. Rupture after endovascular abdominal aortic aneurysm repair: a multicenter study. Vasc Endovascular Surg 2014;48:476-81. [Crossref] [PubMed]
- Santilli SM, Wernsing SE, Lee ES. Expansion rates and outcomes for iliac artery aneurysms. J Vasc Surg 2000;31:114-21. [Crossref] [PubMed]
- Ellozy SH, Carroccio A, Lookstein RA, et al. Abdominal aortic aneurysm sac shrinkage after endovascular aneurysm repair: correlation with chronic sac pressure measurement. J Vasc Surg 2006;43:2-7. [Crossref] [PubMed]
- Patel R, Sweeting MJ, Powell JT, et al. Endovascular versus open repair of abdominal aortic aneurysm in 15-years' follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet 2016;388:2366-74. [Crossref] [PubMed]
- De Bruin JL, Baas AF, Buth J, et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med 2010;362:1881-9. [Crossref] [PubMed]
- Lederle FA, Kyriakides TC, Stroupe KT, et al. Open versus Endovascular Repair of Abdominal Aortic Aneurysm. N Engl J Med 2019;380:2126-35. [Crossref] [PubMed]
- Brin BJ, Busuttil RW. Isolated hypogastric artery aneurysms. Arch Surg 1982;117:1329-33. [Crossref] [PubMed]
- Pruimboom T, Scheltinga M. Massive Buttock Necrosis Following Aortobifemoral Bypass Surgery. Eur J Vasc Endovasc Surg 2018;56:86. [Crossref] [PubMed]
- Sahgal A, Veith FJ, Lipsitz E, et al. Diameter changes in isolated iliac artery aneurysms 1 to 6 years after endovascular graft repair. J Vasc Surg 2001;33:289-4; discussion 294-5. [Crossref] [PubMed]
- Rubenstein C, Bietz G, Davenport DL, et al. Abdominal compartment syndrome associated with endovascular and open repair of ruptured abdominal aortic aneurysms. J Vasc Surg 2015;61:648-54. [Crossref] [PubMed]
- Kirkpatrick AW, Roberts DJ, De Waele J, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 2013;39:1190-206. [Crossref] [PubMed]
- Berry N, Fletcher S. Abdominal compartment syndrome. Contin Educ Anaesthesia, Crit Care Pain 2012;12:110-7. [Crossref]
- De Waele JJ, Hoste EA, Malbrain ML. Decompressive laparotomy for abdominal compartment syndrome--a critical analysis. Crit Care 2006;10:R51. [Crossref] [PubMed]
Cite this article as: Cañizares Otero MC, Osterman F, Danckers M. Delayed complication of iliac artery aneurysm repair—from critical limb ischemia to mixed shock: a case report. AME Case Rep 2022;6:28.


