
Synchronous papillary-medullary thyroid microcarcinoma: a case report
Introduction
The most common type of thyroid carcinoma is papillary thyroid carcinoma, comprising 85–90% of all thyroid carcinomas (1). Medullary thyroid carcinoma, originating from calcitonin-producing parafollicular cells (C-cells), is the third most common type of thyroid carcinoma. It makes up less than 5% of all thyroid carcinomas (1). The micro subtypes of thyroid carcinomas are defined as carcinomas measuring 1 cm or less. Although both papillary and medullary thyroid carcinomas have been well studied in isolation, our knowledge regarding the biology, natural history, and treatment of synchronous papillary-medullary thyroid carcinoma is limited and can pose both diagnostic and management challenges. Here we present a rare case of synchronous papillary-medullary thyroid microcarcinoma and treatment via total thyroidectomy, radioactive iodine ablation, and exogenous hormonal suppression. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-13/rc).
Case presentation
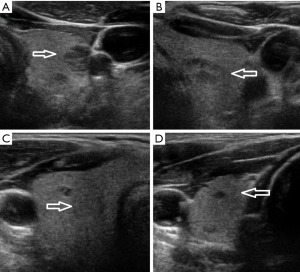
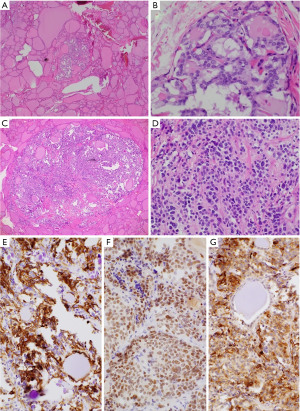
A 65-year-old man presented with an increasingly symptomatic multinodular thyroid goiter that had progressed over the course of years. The patient denied a history of radiation exposure but had a family history of an unknown type of thyroid carcinoma in his mother. Ultrasound (US) examination of the goiter confirmed bilateral thyroid nodules up to ~1.6 cm (Figure 1). Fine needle aspiration (FNA) biopsy of the left dominant nodule showed atypia of undetermined significance (AUS) (Bethesda class III) with atypical microfollicles and follicular cells with nuclear grooves and clearing. He had no mutations detected by the ThyGeNEXT oncogene panel and the ThyraMIR® (microRNA risk classifier) was negative, which then classified the FNA results as very highly likely benign. Because of the progressively worsening local, compressive symptoms after his FNA, the patient underwent a successful total thyroidectomy with intraoperative nerve integrity monitoring. He had an uneventful postoperative recovery course with preserved phonation and parathyroid gland function. The final surgical pathology report indicated a synchronous multicentric multifocal papillary microcarcinoma and medullary microcarcinoma. Pathology analysis showed a multifocal classic papillary microcarcinoma, mpT1aNx [American Joint Committee on Cancer (AJCC)], with two foci in the left lobe (0.2 and 0.1 cm) and two foci in the right lobe (0.1 and 0.5 cm). There was also a unifocal medullary carcinoma, AJCC pT1aNx, 0.3 cm in the left lobe (Figure 2). The specimen showed no extrathyroidal extension, angioinvasion, or lymphovascular invasion. The margins were uninvolved based on the total thyroidectomy specimen. The patient underwent a postoperative radioactive iodine ablation with 30 miCu of radioactivity and was placed on a suppressive dose of exogenous thyroid hormone (levothyroxine). On follow-up 6 months postoperatively, the patient is doing well, disease free with a thyroglobulin level less than 0.1 ng/mL and a calcitonin level less than 2 ng/mL.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Thyroid follicular structures mainly consist of two cell populations: follicular cells which produce colloid and thyroid hormones, and parafollicular cells which produce calcitonin. Follicular cells can give rise to a spectrum of carcinomas ranging from well-differentiated papillary carcinoma to undifferentiated anaplastic thyroid carcinoma. The prognostically-favorable papillary carcinoma has a greater than 99% 10-year survival rate in people 45 years and younger regardless of the stage of the disease (2). On the other hand, anaplastic thyroid carcinoma carries a very poor prognosis with a median survival rate of 4 months (3). Parafollicular cells are neuroendocrine cells that originate from the pharyngeal endoderm and can give rise to medullary carcinoma. Medullary carcinoma can arise sporadically or as part of a familial multiple endocrine neoplasia (MEN) such as MEN 2A, and MEN 2B.
Rarely, two different types of carcinomas can co-occur, such as papillary carcinoma and medullary carcinoma, classified as a synchronous (mixed) papillary-medullary thyroid carcinoma. The literature shows that mixed tumors are most often coalesced in the same tumor bulk (Table 1). This case is very specific, because we describe completely separate synchronous papillary and medullary carcinomas. The origin and biology of synchronous papillary-medullary thyroid carcinomas are not completely understood; however, leading theories include stem cell theory, collision effect theory, and hostage theory (27,28). The stem cell theory suggests that the carcinoma originates from a rare population of cancerous stem cells (29). Collision effect theory postulates that the mixed carcinoma originates as two distinct tumor types that initiate near each other resulting in a polyclonal neoplasm that is recognized as a single entity (27). The “hostage” theory hypothesizes that the carcinoma is a result of adenomatous areas being sequestered by a different type of tumor (28).
Table 1
| Article number | Citation | Number of cases | Histology of thyroid components |
|---|---|---|---|
| 1 | Nangue et al., 2009 (4) | 1 | MTC in the right thyroid lobe, closely intermingled with a nonencapsulated classical PTC |
| 2 | Samarasinghe et al., 2020 (5) | 1 | Multifocal PTC in the left thyroid nodule. MTC and PTC within a lymph node of left lateral neck. MTC in the right lobe |
| 3 | Gurkan et al., 2014 (6) | 2 | Mixed medullary-papillary thyroid carcinoma with co-occurrence of MTC and PTC |
| 4 | Yao et al., 2020 (7) | 1 | PTC in the right lobe and isthmus of the thyroid. MTC in the left lobe |
| 5 | Hasney et al., 2010 (8) | 1 | MTC with a distinct focus of PTC in the left lobe of the thyroid |
| 6 | Jain et al., 2014 (9) | 1 | Mixed medullary-papillary carcinoma of the thyroid |
| 7 | Myoteri et al., 2016 (10) | 1 | Mixed MTC/PTC |
| 8 | Guerreiro et al., 2021 (11) | 1 | Mixed medullary-papillary carcinoma of the thyroid |
| 9 | Shimizu et al., 2000 (12) | 1 | Mixed medullary-follicular carcinoma of the thyroid and PTC with a clear border between the two components |
| 10 | Chambers et al., 2021 (13) | 1 | PTC which transitioned to a morphologically and immunophenotypically distinct MTC component within the same lesion |
| 11 | Kataria et al., 2013 (14) | 1 | Mixed medullary-papillary carcinoma of the thyroid with C-cell hyperplasia |
| 12 | Tang et al., 2017 (15) | 1 | Synchronous multiple discrete MTC and PTC |
| 13 | Wu et al., 1998 (16) | 1 | Mixed medullary-follicular carcinoma of the thyroid with concurrent PTC |
| 14 | Shiroko et al., 2001 (17) | 1 | Mixed medullary-papillary carcinoma in right thyroid |
| 15 | Parker et al., 1985 (18) | 1 | Medullary, papillary, follicular, and undifferentiated carcinoma of the same gland |
| 16 | Dionigi et al., 2007 (19) | 2 | Multicentric MTC and PTC with mixed features found in the isthmus of the gland |
| 17 | Lax et al., 1994 (20) | 3 | In two cases the papillary component was characterized by typical papillae with a fibrovascular core; in one a follicular variant of PTC was found |
| 18 | Macák et al., 1997 (21) | 1 | MTC in the upper part of the right lobe and mixed medullary-papillary carcinoma in the left lobe of the thyroid gland |
| 19 | Seki et al., 2004 (22) | 2 | MTC and PTC separate but synchronous in the thyroid but mixed in some lymph node metastases |
| 20 | Michal et al., 1993 (23) | 2 | Mixed medullary-follicular carcinoma with cytological features of PTC |
| 21 | Gupta, 2013 (24) | 1 | Parathyroid hyperplasia and MTC mixed with PTC |
| 22 | Meshikhes et al., 2004 (25) | 1 | PTC in the right lobe and MTC in the left lobe |
| 23 | Apel et al., 1994 (26) | 1 | Thyroglobulin-positive PTC intermixed with calcitonin-containing MTC |
MTC, medullary thyroid carcinoma; PTC, papillary thyroid carcinoma.
Thyroid carcinomas can be diagnosed in a few different ways, the main ones are physical exam with imaging for confirmation or incidental finding on an US, magnetic resonance imaging (MRI), or computed tomography (CT) scan. Depending on the index of suspicion, thyroid nodules should be biopsied using FNA. For medullary carcinomas calcitonin levels should be assessed. If biopsy reveals medullary carcinoma a priori, then it must be staged due to a high probability of metastasis to the locoregional lymph nodes, lungs, bones, brain. Metastatic disease should be suspected if the calcitonin level is greater than 500 pmol/L (30). Diagnosis is most often made by morphological examination, standard immunohistochemistry studies, and molecular detection based on the biopsy sample. Other diagnostic hints indicating this disease include elevated laboratory values of thyroglobulin, calcitonin, and carcinoembryonic antigen (4,5,9,10,14,17,31).
In this case, the diagnosis was made post-surgically. The patient’s FNA biopsy revealed AUS. Total thyroidectomy was the choice of treatment due to the enlarging goiter with progressively worsening local compressive symptoms. The final surgical pathology report indicated a synchronous multicentric multifocal papillary microcarcinoma and medullary microcarcinoma.
The medullary component is the main prognostic factor in cases of synchronous papillary-medullary thyroid malignancy, and the extent of surgical resection depends on the stage of the tumor (4,5,7,10,32). Although radioactive iodine ablation and thyroid-stimulating hormone (TSH) suppression is not useful in treatment of lone medullary thyroid carcinoma due to lack of accumulation of radioiodine in parafollicular C-cells, it may be useful for the treatment of the co-occurring papillary carcinoma (1,33). In our case, the incidentally found tumors were treated appropriately with simple total thyroidectomy due to the lack of clinically significant lateral neck lymphadenopathy, post-surgical radioactive iodine ablation, and suppressive levels of oral levothyroxine. In addition, the patient will be followed annually with TSH, thyroglobulin, and calcitonin levels to monitor for recurrence, with further imaging if lab results warrant suspicion. Our case follows the management guidelines laid out by the Revised American Thyroid Association Guidelines for the Management of Medullary Thyroid Carcinoma (33).
Synchronous papillary-medullary thyroid microcarcinoma is a rare co-incidental carcinoma that should be treated according to the staging of its components. Although papillary thyroid carcinoma is amenable to radioactive iodine ablation, medullary thyroid carcinoma is not, and therefore synchronous papillary-medullary thyroid microcarcinoma should be treated surgically to reduce the risk of recurrence. They also have different biological effects as the papillary form is derived from thyroid hormone-producing follicular cells, whereas the medullary form is derived from C-cells. The downstream effects can then be used to observe for recurrence by monitoring calcitonin, TSH, and thyroglobulin.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-13/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-22-13/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-13/coif). VKN serves as an unpaid editorial board member of AME Case Reports from February 2022 to January 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu FC, Lin HT, Lin SF, et al. Nationwide cohort study on the epidemiology and survival outcomes of thyroid cancer. Oncotarget 2017;8:78429-51. [Crossref] [PubMed]
- Adam MA, Thomas S, Hyslop T, et al. Exploring the Relationship Between Patient Age and Cancer-Specific Survival in Papillary Thyroid Cancer: Rethinking Current Staging Systems. J Clin Oncol 2016;34:4415-20. [Crossref] [PubMed]
- Nagaiah G, Hossain A, Mooney CJ, et al. Anaplastic thyroid cancer: a review of epidemiology, pathogenesis, and treatment. J Oncol 2011;2011:542358. [Crossref] [PubMed]
- Nangue C, Bron L, Portmann L, et al. Mixed medullary-papillary carcinoma of the thyroid: report of a case and review of the literature. Head Neck 2009;31:968-74. [Crossref] [PubMed]
- Samarasinghe S, Yuksel S, Mehrotra S. Intermixed medullary and papillary thyroid cancer in a patient with renal cell carcinoma. Endocrinol Diabetes Metab Case Rep 2020; Epub ahead of print. [Crossref] [PubMed]
- Gurkan E, Gurbuz Y, Tarkun I, et al. Mixed medullary-papillary carcinoma of the thyroid: report of two cases and review of the literature. Indian J Pathol Microbiol 2014;57:598-602. [Crossref] [PubMed]
- Yao J, Li CF, Wang JJ, et al. Medullary thyroid carcinoma combined with papillary thyroid carcinoma: case report and literature review. Int J Clin Exp Pathol 2020;13:2710-7. [PubMed]
- Hasney CP, Amedee RG. Mixed medullary-papillary carcinoma of the thyroid: a case report. Laryngoscope 2010;120:S153. [Crossref] [PubMed]
- Jain M, Verma D, Thomas S, et al. Mixed medullary - papillary carcinoma thyroid: an uncommon variant of thyroid carcinoma. J Lab Physicians 2014;6:133-5. [Crossref] [PubMed]
- Myoteri D, Dellaportas D, Carvounis E, et al. Mixed medullary and papillary thyroid carcinoma: A stepwise diagnosis. J BUON 2016;21:1561-2. [PubMed]
- Guerreiro V, Costa C, Oliveira J, et al. Mixed medullary-papillary thyroid carcinoma with mixed lymph node metastases: A case report. Clin Case Rep 2021;9:e04165. [Crossref] [PubMed]
- Shimizu M, Hirokawa M. Combined "Mixed Medullary-Follicular" and "Papillary" Carcinoma of the Thyroid with Lymph Node Metastasis. Endocr Pathol 2000;11:353-8. [Crossref] [PubMed]
- Chambers M, Tafe LJ, Gutmann EJ, et al. Cytologic features of a case of mixed medullary and follicular cell-derived thyroid carcinoma with review of the literature. Diagn Cytopathol 2021;49:E125-9. [Crossref] [PubMed]
- Kataria K, Yadav R, Sarkar C, et al. Simultaneous medullary carcinoma, papillary carcinoma and granulomatous inflammation of the thyroid. Singapore Med J 2013;54:e146-8. [Crossref] [PubMed]
- Tang PY, Khor LY, Takano A. Synchronous papillary thyroid carcinoma and medullary thyroid carcinoma - a pitfall waiting to happen. Malays J Pathol 2017;39:171-4. [PubMed]
- Wu CJ, Chen HL, Song YM, et al. Mixed medullary-follicular carcinoma and papillary carcinoma of the same thyroid. Intern Med 1998;37:955-7. [Crossref] [PubMed]
- Shiroko T, Yokoo N, Okamoto K, et al. Mixed medullary-papillary carcinoma of the thyroid with lymph node metastases: report of a case. Surg Today 2001;31:317-21. [Crossref] [PubMed]
- Parker LN, Kollin J, Wu SY, et al. Carcinoma of the thyroid with a mixed medullary, papillary, follicular, and undifferentiated pattern. Arch Intern Med 1985;145:1507-9. [Crossref] [PubMed]
- Dionigi G, Castano P, Bertolini V, et al. Simultaneous medullary and papillary thyroid cancer: two case reports. J Med Case Rep 2007;1:133. [Crossref] [PubMed]
- Lax SF, Beham A, Kronberger-Schönecker D, et al. Coexistence of papillary and medullary carcinoma of the thyroid gland-mixed or collision tumour? Clinicopathological analysis of three cases. Virchows Arch 1994;424:441-7. [Crossref] [PubMed]
- Macák J, Vaverková H, Dushová M, et al. Medullary and mixed medullary-papillary carcinoma of the thyroid gland. Pathologica 1997;89:317-22. [PubMed]
- Seki T, Kameyama K, Hayashi H, et al. Composite metastatic carcinoma in lymph nodes of patients with concurrent medullary and papillary thyroid carcinoma: a report of two cases. Endocr Pathol 2004;15:83-8. [Crossref] [PubMed]
- Michal M, Curík R, Macák J, et al. Mixed medullary-follicular and medullary-papillary carcinoma of the thyroid: one or two entities? Zentralbl Pathol 1993;139:333-5. [PubMed]
- Gupta V. Simultaneous presentation of giant pheochromocytoma, primary hyperparathyroidism, and mixed-medullary-papillary thyroid cancer in MEN 2A. Indian J Endocrinol Metab 2013;17:751-5. [Crossref] [PubMed]
- Meshikhes AW, Tingura M, Al-Saeed JY. Concurrent papillary and medullary thyroid carcinomas with mixed metastases to lymph nodes. Saudi Med J 2004;25:373-5. [PubMed]
- Apel RL, Alpert LC, Rizzo A, et al. A metastasizing composite carcinoma of the thyroid with distinct medullary and papillary components. Arch Pathol Lab Med 1994;118:1143-7. [PubMed]
- Pishdad R, Cespedes L, Boutin R, et al. Coexistence of Two Different Thyroid Malignancies: A Collision Phenomenon. Cureus 2020;12:e7539. [Crossref] [PubMed]
- Bangaraiahgari R, Panchangam RB, Puthenveetil P, et al. Is there adenoma-carcinoma sequence between benign adenoma and papillary cancer of thyroid: A genomic linkage study. Ann Med Surg (Lond) 2020;60:695-700. [Crossref] [PubMed]
- Yoo MH, Hatfield DL. The cancer stem cell theory: is it correct? Mol Cells 2008;26:514-6. [PubMed]
- Opsahl EM, Akslen LA, Schlichting E, et al. The Role of Calcitonin in Predicting the Extent of Surgery in Medullary Thyroid Carcinoma: A Nationwide Population-Based Study in Norway. Eur Thyroid J 2019;8:159-66. [Crossref] [PubMed]
- Monden T, Mori M. Many faces of mixed medullary-follicular and papillary carcinoma of the thyroid. Intern Med 1998;37:909-10. [Crossref] [PubMed]
- Goyal R, Nada R, Rao KL, et al. Mixed medullary and follicular cell carcinoma of the thyroid with lymph node metastasis in a 7-year-old child. Pathol Int 2006;56:84-8. [Crossref] [PubMed]
- Wells SA Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015;25:567-610. [Crossref] [PubMed]
Cite this article as: Medam RR, Castro G, Alhassan R, Neychev VK. Synchronous papillary-medullary thyroid microcarcinoma: a case report. AME Case Rep 2022;6:27.


