
Migration of Calypso beacon transponders for hepatic stereotactic body radiotherapy: a report of two cases
Introduction
Stereotactic body radiation therapy (SBRT) delivers a highly conformal radiation dose to the target lesion while keeping dose received by neighboring critical structures to a minimum (1).
The conformality of SBRT allows the delivery of ablative dose of radiation to the target with a rapid dose fall off outside. Liver cancers move with breathing cycles. Liver motion with breathing could be variable posing a difficult challenge to delivering the required high radiation dose while staying safe with regard to surrounding normal tissues.
A large internal target volume (ITV) is required when treating a mobile target in free breathing. The large ITV is mandatory to encompass the target in all phases of breathing cycle (2). Consequently, a large planning target volume (PTV) is required with increased risk of normal tissue toxicity. Different techniques have been developed to reduce the volume of normal organs within the treated volume. One technique is respiratory gating through which radiation is delivered during only a portion of the respiratory cycle, consequently the ITV is reduced (3). Implanted fiducial markers work as surrogates for the target and allow for real time tracking of target motion. Tracking of markers can be combined with respiratory gating (4). However, some risks associated with insertion of markers have been observed.
A retrospective review by Morita et al. (5) evaluated 116 percutaneous intrahepatic implantations of gold fiducial markers found 7 markers to have migrated out of the liver and 5 were found in intrahepatic vessels which occurred without complications and those markers were not retrieved. Other serious migration cases have been reported including migration to the right atrium (6) and to coronary artery that led to a myocardial infarction after insertion of markers in liver (7).
Calypso tracking system with Dynamic EdgeTM gating (from Varian Medical Systems) tracks motion of electromagnetic transponders that are dependent on delivery of any additional radiation to the patient. It requires 2–3 electromagnetic transponders (beacons) to be implanted in or close to the target. For soft tissue (prostate and liver), Calypso beacons measure 1.3 mm in diameter and 8.7 mm in length (Figure 1A). Anchored Calypso beacons (Figure 1B) are typically used for lung implants. Calypso system was commissioned for clinical use after completing all the validation checks for localization and tracking. We routinely use Calypso beacons for liver, prostate and lung tumors treated with SBRT. Each beacon reflects an electromagnetic frequency unique to it. The centroid of the beacons is used to calculate the position of the target in real time (9).
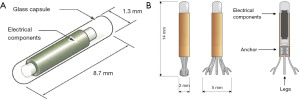
The user Beacon care package contains three single-use 17-gauge introducer needles (that are numbered and colour-coded) and beacon transponders for implantation. Each transponder is manually loaded into the 17-gauge introducer for implantation. The Calypso System has been validated for providing real-time target tracking of cancers in a variety of solid organs (10).
Here, we present two cases of migration of Calypso soft tissue transponders to the lung shortly after implantation in liver. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-6/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Patient A
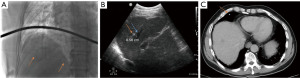
A 40-year-old male with hepatocellular carcinoma (HCC) in segment 8 of liver with invasion of branch portal vein but no portal hypertension was referred for consideration of SBRT. Under ultrasound (US) guidance, 2 Calypso beacons were implanted superior and inferior to the lesion. Post insertion fluoroscopic images confirmed the position of the beacons (Figure 2A). The medially located marker was inserted 0.56 cm away from a branch middle hepatic vein (Figure 2B). CT simulation was performed 4 days post insertion and showed only one marker in liver while the 2nd (medially placed fiducial seen on fluoroscopic image in Figure 2A) had migrated to the right lung (Figure 2C). Patient was completely asymptomatic. Two other beacons were inserted, and SBRT was delivered uneventfully.
Patient B
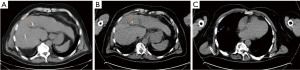
A 69-year-old male with multifocal HCC and advanced portal hypertension was referred for liver SBRT with disease progression after transarterial chemoembolization (TACE). The target lesion was located centrally within segment V/VI. Respiratory gated SBRT using Calypso soft tissue transponders was planned. Three Calypso beacons were inserted under CT guidance superior, anterior and posterior to the lesion. An immediate post procedure CT scan confirmed the locations of the beacons (Figure 3A). A previously planned PET CT scan was performed 2 days after the insertion which showed only 2 beacons in place. The posterior marker was not identified in liver (Figure 3B). The migrated posterior Calypso beacon, which was inserted 1–1.5 cm from a branch right hepatic vein, was identified in the right lung (Figure 3C). Patient was asymptomatic and treatment was delivered uneventfully with respiratory gating using only 2 beacons.
Discussion
Fiducial markers have been shown to improve precision of radiotherapy delivery as surrogates for tumor tracking. Fiducial markers designed for image guided technologies should be inert, and be properly visualized when inserted into target organ. The markers should be small in diameter, to allow for easy insertion with small range needles (11). Migration of regular fiducial markers, non-Calypso, from its original site of insertion has been reported (5). Migration will affect the accuracy of radiation delivery and carries risk of potential damage to the organ where migration occurred (12).
Here we report, for the first time, on 2 cases where Calypso soft tissue transponders migrated to right lung shortly after implantation. Two reasons are postulated to explain fiducial migration. First reason could be the casing of the beacon transponders. The inner metal core is covered by a shell made of glass, which is very smooth and makes it easy to travel away from the insertion site specially in liver where tissue boundaries can be easily breached. Other types of cylindrical shaped fiducials showed increased risk of migration because their homogenous shape tends to allow them to move easily (13). Second reason could be the location of insertion, where Calypso markers might have been implanted close to a branch hepatic vein. The proximity to a blood vessel with a brisk blood flow might lead to instability in the location of the marker and ultimately its migration.
We recommend maintaining a safe distance from any branch hepatic vein to the planned location of the beacon. It is not clear how far exactly, but a minimum of one centimeter is probably a safe distance which is fractionally larger than the maximum length of the soft tissue beacon (8.7 mm). Moreover, if the planned insertion site is close to a blood vessel (as assessed in the pre-insertion infused CT scan or better by an US Doppler), we suggest implanting Calypso beacons under US-guidance after Doppler assessment. Through the Doppler examination, the blood flow can be detected and insertion could be planned to be safely away from the vessels and thus reduce the risk of migration. Alternatively, “volume navigation” which is a real-time image-fusion technology could be considered to ensure accurate positioning of Calypso beacons under US-guidance (14). Finally, while anchored Calypso beacons (Figure 1B) are approved for insertion in lung (8), one might study the possibility of inserting them in liver as well. Those beacons measure 2 mm in diameter and 8 mm in length (14 mm including the anchoring legs). In order to avoid the migration of the beacon within the airways, the beacon has an attached five-legged anchoring system that expands to 5 mm in diameter once deployed. This mechanism could potentially be very useful for better anchoring in the liver.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-6/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-22-6/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-6/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Scorsetti M, Clerici E, Comito T. Stereotactic body radiation therapy for liver metastases. J Gastrointest Oncol 2014;5:190-7. [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Yoganathan SA, Maria Das KJ, Agarwal A, et al. Magnitude, Impact, and Management of Respiration-induced Target Motion in Radiotherapy Treatment: A Comprehensive Review. J Med Phys 2017;42:101-15. [Crossref] [PubMed]
- Habermehl D, Henkner K, Ecker S, et al. Evaluation of different fiducial markers for image-guided radiotherapy and particle therapy. J Radiat Res 2013;54:i61-8. [Crossref] [PubMed]
- Morita R, Abo D, Sakuhara Y, et al. Percutaneous insertion of hepatic fiducial true-spherical markers for real-time adaptive radiotherapy. Minim Invasive Ther Allied Technol 2020;29:334-43. [Crossref] [PubMed]
- Khullar K, Dhawan ST, Nosher J, et al. Fiducial marker migration following computed tomography-guided placement in the liver: a case report. AME Case Rep 2021;5:15. [Crossref] [PubMed]
- Hennessey H, Valenti D, Cabrera T, et al. Cardiac embolization of an implanted fiducial marker for hepatic stereotactic body radiotherapy: a case report. J Med Case Rep 2009;3:140. [Crossref] [PubMed]
- Varian Medical Systems. Beacon care package-lung, Instructions for use. 2014.
- Varian Medical Systems. Calypso soft tissue (17G) Beacon transponder. 2016.
- D’Ambrosio DJ, Bayouth J, Chetty IJ, et al. Continuous localization technologies for radiotherapy delivery: Report of the American Society for Radiation Oncology Emerging Technology Committee. Pract Radiat Oncol 2012;2:145-50. [Crossref] [PubMed]
- O’Neill AG, Jain S, Hounsell AR, et al. Fiducial marker guided prostate radiotherapy: a review. Br J Radiol 2016;89:20160296. [Crossref] [PubMed]
- Kitamura K, Shirato H, Shimizu S, et al. Registration accuracy and possible migration of internal fiducial gold marker implanted in prostate and liver treated with real-time tumor-tracking radiation therapy (RTRT). Radiother Oncol 2002;62:275-81. [Crossref] [PubMed]
- Kim SH, Shin EJ. Endoscopic Ultrasound-Guided Fiducial Placement for Stereotactic Body Radiation Therapy in Pancreatic Malignancy. Clin Endosc 2021;54:314-23. [Crossref] [PubMed]
- Tokunaga K, Furuta A, Iizuka Y, et al. Utility of real-time image fusion technology in ultrasonography-guided fiducial marker implantation for stereotactic body radiation therapy for liver tumors. Acta Radiol 2021;62:567-73. [Crossref] [PubMed]
Cite this article as: Amjad R, Soliman Y, Pereira M, Cassano-Bailey A, Vivian M, Venkataraman S, Nashed M. Migration of Calypso beacon transponders for hepatic stereotactic body radiotherapy: a report of two cases. AME Case Rep 2022;6:25.


