
Combined portable cone beam computed tomography and robotic-assisted bronchoscopy impacting diagnosis of a solitary pulmonary nodule: a case report
Introduction
Lung cancer is the leading cause of cancer-related mortality in men and women throughout the world and accounts for 23% of cancer deaths in the US (1-3). To detect lung cancer at an earlier stage, an annual computed tomography (CT) chest is recommended for at-risk individual screening which has led to an increased detection of pulmonary nodules (4). Bronchoscopy historically has played a minor role in pulmonary nodule management due to a limited diagnostic accuracy of 14% to 31% (5). With the emergence of robotic bronchoscopy, biopsy yields have been reported from 69.1% to 81.7% (6-9). However, when diaphragmatic movement distorts nodule positioning and alters the “virtual target” derived from preprocedural CT navigation, reduced diagnostic yield, added procedural time, and possible complications can occur in a phenomenon known as “CT-to-body divergence” (10).
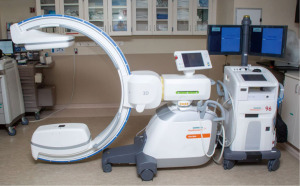
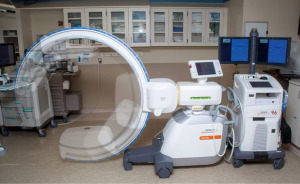
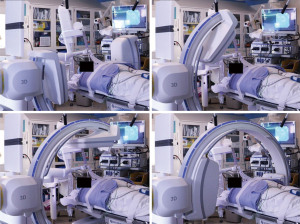
Cone beam computed tomography (CBCT) with fixed c-arms can eliminate divergence by generating a CT-like image in one single, 4–10 seconds, intraprocedural rotation around the patient (11). Improved targeting accuracy and low radiation dose have been seen in studies utilizing CBCT (12). Housing a fixed system in a designated operating room can be cost-prohibitive and interfere with equipment positioning (11). The introduction of portable three-dimensional (3D) fluoroscopy has overcome these hurdles. The CIOS 3D Spin Mobile (Siemens Healthineers, Malvern, PA, USA; Figure 1) is compact, mounted on a C-arm, and produces CT images to confirm positioning of biopsy tools prior to sampling as demonstrated in the following case. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-5/rc).
Case presentation
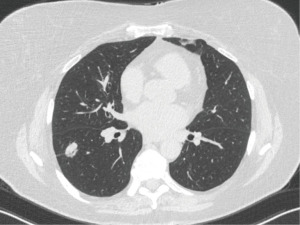
A 72-year-old nonsmoker with a history of melanoma presented for nodule evaluation. While the patient was asymptomatic, a routine CT scan for cancer surveillance demonstrated an incidentally found 2.1-cm solid nodule in the right lower lobe that was hypermetabolic on subsequent positron emission tomography (PET) imaging, suspicious for metastasis versus primary lung cancer (Figure 2). There was an air bronchus sign present. Her physical exam was unremarkable except for stable skin findings from the previously diagnosed melanoma, and she denied relevant medical, family, and psycho-social history.
The patient was enrolled in a clinical trial utilizing combination robotic bronchoscopy and CIOS and consented to participate (NCT04740047). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
The patient was intubated for the procedure, and the ventilator was sent to tidal volumes between 6 to 8 mL/kg of ideal body weight, with a predisposition for higher tidal volumes and lower respiratory rates. Given the location of the lower lobe lesion, positive end-expiratory pressure (PEEP) was set to 15 cmH2O. The lesion of interest was placed in the center of the c-arm by adjusting the table and/or c-arm position using lasers from the c-arm (Figure 3, Video 1). The CIOS was rotated 90° left anterior oblique to 90° right anterior oblique to ensure clearance of all equipment (Figure 4).
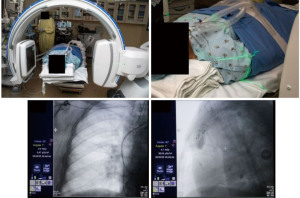
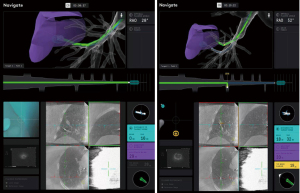
Using the controller and navigation guidance, the proceduralist drove the catheter under live visualization to the target. A 30 second intraoperative CBCT scan with an inspiratory hold at 20 cmH2O visualized the catheter tip relative to the target nodule (Video 2, Figure 5). Measurements were obtained in three axes, including distance to the nodule and the catheter orientation, which showed the tool 18 mm inferior to the nodule. Re-registration and re-navigation showed tool-in-lesion on repeat spin with radial endobronchial ultrasound (EBUS) confirming proximity to the lesion (Figure 6). Biopsies taken were immediately available for pathology review which consisted of a 23-gauge needle and forceps, both of which demonstrated adenocarcinoma with papillary features on the first pass. The total time for the robotic procedure was 18 minutes from docking to undocking. The lymph node pathology from 4R and 11R was negative for malignancy, and she proceeded with lobectomy due to the nodule margins with a favorable prognosis. There were no adverse or unanticipated results. She is currently being observed for reoccurrence by her surgical oncology team.
Discussion
This case demonstrates how the novel portable 3D imaging modality can complement robotic bronchoscopy and alter the diagnostic accuracy of a biopsy. The robotic bronchoscope provides information on the proximity and orientation to critical structures with the CIOS imaging contributing information on spatial relationships between the catheter, airway, and nodule. This combination enables position refinement and confirmation of tool-in-lesion which is important when distinguishing sampling error from a true negative in benign nodule biopsy. In addition, the CIOS can be positioned around the patient with no effect on the proceduralist’s or anesthesiologist’s workspace, can be used in any room, and appears to have a low radiation profile.
The CIOS system has been previously described in limited case series in the literature. Avasarala et al combined electromagnetic navigation with CIOS and found a tool-in-lesion rate of 100% of procedures. Despite this, there was no correlation to increased diagnostic yield (13). Kalchiem-Dekel et al. reported their experience with 10 lesions in 5 patients using Shape-sensing robotic-assisted bronchoscopy (SSRAB) in conjunction with the CIOS Mobile 3D spin. In 90% of cases, tool-in-lesion was captured, and the relationship of the biopsy tool and lesion was improved in 3 cases (30%) based on direct information obtained from the intraoperative imaging (8).
The diagnosis was obtained for this patient utilizing the novel combined technique of robotic peripheral pulmonary nodule biopsy with portable 3D imaging, which may improve overall diagnostic accuracy of bronchoscopic biopsy. Further clinical trials are warranted to assess utility of robotic bronchoscopy and 3D planar imaging.
Conclusions/take-away
The diagnosis of peripheral pulmonary nodules may be improved utilizing the novel combination of robotic bronchoscopy with portable 3D imaging to verify tool-in-lesion prior to biopsy.
Acknowledgments
Siemens provided the equipment as a loaner for a clinical study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-5/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-5/coif). JR has an independent research grant with intuitive surgical that is outside the scope of this work, and receives equipment loan for a clinical study from Siemens. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. An Update on Cancer Deaths in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Division of Cancer Prevention and Control; 2022.
- US Preventive Services Task Force. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325:962-70. [Crossref] [PubMed]
- Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000;117:1049-54. [Crossref] [PubMed]
- Chaddha U, Kovacs SP, Manley C, et al. Robot-assisted bronchoscopy for pulmonary lesion diagnosis: results from the initial multicenter experience. BMC Pulm Med 2019;19:243. [Crossref] [PubMed]
- Fielding DIK, Bashirzadeh F, Son JH, et al. First Human Use of a New Robotic-Assisted Fiber Optic Sensing Navigation System for Small Peripheral Pulmonary Nodules. Respiration 2019;98:142-50. [Crossref] [PubMed]
- Kalchiem-Dekel O, Fuentes P, Bott MJ, et al. Multiplanar 3D fluoroscopy redefines tool-lesion relationship during robotic-assisted bronchoscopy. Respirology 2021;26:120-3. [Crossref] [PubMed]
- Chen AC, Pastis NJ Jr, Mahajan AK, et al. Robotic Bronchoscopy for Peripheral Pulmonary Lesions: A Multicenter Pilot and Feasibility Study (BENEFIT). Chest 2021;159:845-52. [Crossref] [PubMed]
- Pritchett MA, Bhadra K, Calcutt M, et al. Virtual or reality: divergence between preprocedural computed tomography scans and lung anatomy during guided bronchoscopy. J Thorac Dis 2020;12:1595-611. Erratum in: J Thorac Dis 2020 Aug;12(8):4593-4595. [Crossref] [PubMed]
- Cheng GZ, Liu L, Nobari M, et al. Cone beam navigation bronchoscopy: the next frontier. J Thorac Dis 2020;12:3272-8. [Crossref] [PubMed]
- Casal RF, Sarkiss M, Jones AK, et al. Cone beam computed tomography-guided thin/ultrathin bronchoscopy for diagnosis of peripheral lung nodules: a prospective pilot study. J Thorac Dis 2018;10:6950-9. [Crossref] [PubMed]
- Avasarala SK, Machuzak MS, Gildea TR. Multidimensional Precision: Hybrid Mobile 2D/3D C-Arm Assisted Biopsy of Peripheral Lung Nodules. J Bronchology Interv Pulmonol 2020;27:153-5. [Crossref] [PubMed]
Cite this article as: Duke JD, Fernandez-Bussy S, Reisenauer J. Combined portable cone beam computed tomography and robotic-assisted bronchoscopy impacting diagnosis of a solitary pulmonary nodule: a case report. AME Case Rep 2022;6:23.


