
Gastric perforation during second intragastric balloon treatment: a case report
Introduction
Intragastric balloon (IGB) is a non-operative, temporary treatment for obesity. IGBs are introduced into the stomach through an endoscopic procedure, which is subsequently inflated with saline or air, depending on the type of device. Its function is to reduce gastric capacity and increase the sensation of satiety, thereby inducing weight loss. The treatment should have a maximum duration of 6–12 months, depending on the type of balloon, due to increased risk of deflation and migration of the balloon after this point (1). Reviews (2-4) show a significant weight loss during the treatment period, but the patients tend to gain weight after removal of the balloon (1), giving a modest total efficiency (2,5). The most common side effects are nausea and vomiting (23.3%), abdominal pain (19.9%) and gastro-esophageal reflux disease (GERD) (14.4%). More severe complications include gastric ulceration (0.3%), intestinal obstruction (0.8%), gastric perforation (0.1%) and death (0.05%) (4). This case report describes a rare case of gastric perforation, occurring during the second, consecutive IGB treatment.
We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-21-64/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
We report a case of a 54-year-old woman without former medical history or abdominal surgeries, with a BMI of 34 kg/m2, having a gastric perforation after a second treatment with an Orbera® IGB.
The patient had an Orbera® IGB for 12 months, experiencing no side effects, resulting in a total weight loss of 37 kg. She was treated with high dose proton pump inhibitor, though only 5 days a week on the patient’s own initiative, during the whole IGB treatment period. She did not use any nonsteroidal anti-inflammatory drugs (NSAIDs) during the 12 months. Due to the resulting weight loss and the lack of side effects, the patient wanted to repeat the treatment. She had the first IGB removed endoscopically at a private hospital, during which esophagitis and gastritis was detected. Because of this, the insertion of a new IGB was postponed. Nine days after the removal of the first IGB, a gastroscopy was again performed at the same private hospital, finding regression of the gastritis and esophagitis, and a new Orbera® IGB was inserted. After the insertion, the patient was treated with proton pump inhibitor and antiemetics. The patient experienced discomfort in the epigastric region and nausea after the procedure. Twenty-four hours after the insertion, her nausea worsened resulting in extensive vomiting. This led to her admission at the emergency department 2 days after insertion of the second IGB. On admission, objective examination showed slight tenderness in the epigastric region on palpation, normal laboratory tests and no fever. On suspicion of migration of the IGB, an X-ray of the abdomen was performed (Figure 1), showing correct placement of the IGB and was evaluated to be without free air in the abdomen. If we take a closer look at Figure 1, it reveals free air under the left diaphragm, indicated by the solid arrow in Figure 1, even though it was interpreted to be without, in the initial evaluation. The patient probably already had a perforation on admittance, even though it was not detected until later.
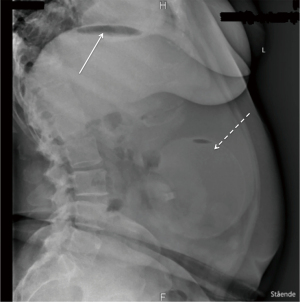
The patient was admitted for observation, and treated with intravenous (i.v.) fluid, painkillers, antiemetics and proton pump inhibitor to relieve the symptoms.
Acute, severe pain in the central abdomen was developed 31 hours after admission, while the patient was still in the hospital. An acute CT scan was performed, showing free air and fluid in the abdomen (Figure 2). The patient underwent an acute gastroscopy showing that the IGB was displaced and stuck in the corpus and antrum part of the stomach. The IGB was punctured, 500 mL of water was extracted and the IGB was removed endoscopically. Esophagitis was detected in the lower 2/3 of the esophagus. The fundus wall was not optimally displayed due to stomach content. The rest of the gastric and duodenal mucosa were found normal. A laparoscopy was then performed, during which a perforation of 1 cm at the top of the gastric fundus was found, surrounded by a hemorrhagic, but viable gastric wall. The abdominal cavity had 2.5 liters of gastric juice and severe peritonitis. The perforation was sutured with continuous sutures, an omental patch was applied over the closure site and peritoneal lavage was performed. An abdominal drain was placed close to the perforation site. A combined ventricular-duodenal probe was placed through a nasal scope. The patient was treated with broad-spectrum i.v. antibiotics and stayed at the intensive care unit for 36 hours, after which she was transferred to the surgical ward and resumed gradual oral feeding.

Postoperatively, the patient developed fever and increasing C-reactive protein (CRP) and leukocytes. On the 9th postoperative day, a CT scan showed multiple intraabdominal abscesses. Two of them were available for ultrasonic drainage, and one in fossa Douglassi was punctured transvaginal.
On the 22nd post-operative day, a new CT scan was performed due to increasing CRP and leukocytes, showing increasing size of the abscess in fossa Douglass, and multiple small abscesses in the abdomen unavailable for drainage. A new transvaginal puncture of the abscess in fossa Douglassi was performed. Her condition improved, and she was discharged on the 28th post-operative day, continuing per oral (p.o.) antibiotics for 1 week.
The patient was readmitted on the 57th postoperative day with fever and abdominal pain. A CT scan showed 8 intra-abdominal abscesses. Compared to the latest CT scan, 2 of these abscesses were new, 3 abscesses had progression, and 3 abscesses had regression. Only 1 of them was available for ultrasonic drainage, which was performed and subsequently she was treated with i.v. antibiotics. The patient was discharged on the 70th postoperative day and her condition was monitored weekly with blood test controls. On the 83rd postoperative day the patient was readmitted due to fever and stomach pain. CT scan showed unchanged conditions intraabdominally since the last CT scan. She underwent vaginal puncture of the abscess in fossa Douglassi, ultrasonic drainage of 3 other abscesses, and was treated with i.v. antibiotics.
On the 92nd postoperative day she was discharged with p.o. antibiotics for 10 days, weekly controls with blood tests and telephonic consultations with a doctor. Her condition improved, and her final follow-up was 132 days after her surgery, where she was free of symptoms. An overview of the patient’s course is presented in a timeline in Figure 3.
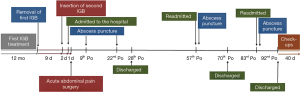
Discussion
Gastric perforation is a rare, but severe complication to IGB (4). The mechanisms behind these perforations are not well described. It is suggested that the IGB can cause excessive pressure on the gastric wall, with subsequent erosion, ulceration and perforation (6,7). A review by Caruso et al. (8), found 21 cases with perforation after IGB treatment, between 2001 and 2018. The onsets of the perforations were ranging from 2 hours after the IGB insertion, to 22 months after. Only 3 of the perforations occurred under 10 days after insertion. Five of the cases in the study had exceeded the recommended treatment time. Eight of the patients had former gastric surgery, which is now recommended to be an absolute contraindication for IGB treatment (9,10). It is recommended that the Orbera® IGB is removed after 6 months (11). The patient in our case report had an Orbera® IGB for 12 months before inserting a second shortly after, thereby exceeding the recommended treatment period. The prolonged treatment period might have resulted in excessive pressure on the gastric wall and gastritis.
We have only found one other case in literature where perforation has occurred during the second IGB treatment. Abou Hussein et al. (12) reported a perforation 3 months after insertion of a second IGB. The patient had an interval of almost 1 year between the first and second IGB treatment. Studies have shown that patients treated with a second IGB, experience a greater short term BMI reduction compared to individuals without a second IGB treatment, though no significant difference is found at long term follow-up. In patients with a second IGB or prolonged IGB use, risks and complications tend to be more frequent (13,14). Active gastritis and severe esophagitis are considered relative contraindications to IGB treatment (15). The patient did not take high dose proton pump inhibitor every day as subscribed, but only 5 days a week. This can have contributed to her gastritis and esophagitis detected upon removal of the first IGB. Regression of gastritis was found before placing the new IGB, but 9 days is not enough time to heal gastritis, even though it macroscopically no longer was visible. The stomach mucosa had been affected and might have been in bigger risk of perforation. With a perforation occurring shortly after endoscopy, one could also suspect that mucosal lesions were overlooked. Insertion of the second IGB should have been postponed.
A study by Joffe et al. found significant macroscopical and histological inflammation in the stomach after IGB removal, and also found that the stomach mucosa took 14 days to normalize. Thus, it is recommended that radical bariatric surgery is not performed until 14 days after removal (16). Another study revealed diffuse mucosal inflammatory infiltrate in the stomach mucosa 6 months after IGB removal, in patients where pre-IGB biopsies were normal (17). There is no standard recommendation for the interval between two IGB treatments. Due to the fact that stomach mucosa is found inflamed, also histologically, one could suggest that a minimum of 2 weeks between two IGB treatments should be implemented, as when bariatric surgery is performed post IGB treatment, to allow recovery of the gastric mucosa. The stomach mucosa might even need longer time to fully recover, as inflammation is also found 6 months after IGB removal.
Second IGB treatments are associated with higher rates of complications (13,14). There are not enough detailed studies about the type and percentage of complications that occur during the second IGB. A theory could be that a full recovery of the gastric mucosa, before insertion of a second IGB, also could decrease the total amount of complications, not only perforation.
The patient’s perforation could be the combined result of the long treatment period and gastritis, supporting the theory of excessive pressure on the gastric wall leading to perforations. The IGB was found displaced to the antrum and corpus part of the stomach during surgery. The displacement might also have led to excessive pressure on one part of the stomach mucosa, even though the perforation was found in the fundus and not in the antrum or corpus. The perforation occurred shortly after insertion of the second IGB, so the insertion procedure could also have been a contributing factor to the perforation. This report lacks information about the insertion procedure due to the fact that it was performed at a private hospital, and we cannot access the journal. This could have given more information on why the IGB treatment led to perforation.
Pneumoperitoneum under the diaphragm was not detected on admission. The patient had, most likely, already a perforation on admission. It is highly likely that the delayed diagnosis contributed to the severe peritonitis detected during surgery, and her recurring abscesses. Barrichello Junior et al. have reported 3 cases of gastric perforation caused by IGB, where an early diagnosis made it possible to successfully treat the perforation with endoscopic closure and endoclips (18). A fast diagnosis with minimal invasive treatment could have resulted in a short, eventless postoperative course. Thus, we recommend that a CT scan should always be performed if a patient is admitted with serious abdominal pain shortly after an IGB insertion.
Treating patients with consecutive IGBs should be done with carefulness, especially when patients have a history of gastritis. The recommended treatment period should not be exceeded. Caution is warranted if a new IGB is inserted closely after the removal of another IGB. Intraabdominal perforations should always be suspected in patients with an IGB, presenting with acute symptoms of pain and vomiting.
Patient perspective: My first IGB was a great success and I experienced very few side effects. Therefore, I was excited to get a new IGB to continue my weight loss. I had not imagined that I could be so sick from this treatment. I had a long course with intensive care and continuing symptoms for months. One year after my surgery, I’ve still not regained my full strength and energy. I will never get an IGB again.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-21-64/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-21-64/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-21-64/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fernandes M, Atallah AN, Soares BG, et al. Intragastric balloon for obesity. Cochrane Database Syst Rev 2007;CD004931. [PubMed]
- Tate CM, Geliebter A. Intragastric Balloon Treatment for Obesity: Review of Recent Studies. Adv Ther 2017;34:1859-75. [Crossref] [PubMed]
- Kim SH, Chun HJ, Choi HS, et al. Current status of intragastric balloon for obesity treatment. World J Gastroenterol 2016;22:5495-504. [Crossref] [PubMed]
- Yorke E, Switzer NJ, Reso A, et al. Intragastric Balloon for Management of Severe Obesity: a Systematic Review. Obes Surg 2016;26:2248-54. [Crossref] [PubMed]
- Gollisch KSC, Raddatz D. Endoscopic intragastric balloon: a gimmick or a viable option for obesity? Ann Transl Med 2020;8:S8. [Crossref] [PubMed]
- Bekheit M, Abdelsalam WN, Sgromo B, et al. Is conservative management for gastric perforation secondary to intragastric balloon possible? Case report and review of literature. Obes Surg 2014;24:968-70. [Crossref] [PubMed]
- Charalambous MP, Thompson J, Efthimiou E. Late gastric perforation after insertion of intragastric balloon for weight loss--video case report and literature review. Surg Obes Relat Dis 2012;8:121-3. [Crossref] [PubMed]
- Caruso R, Vicente E, Quijano Y, et al. A Combined Laparoscopic and Endoscopic Approach for an Early Gastric Perforation Secondary to Intragastric Balloon: Endoscopic and Surgical Skills with Literature Review. Obes Surg 2020;30:4103-6. [Crossref] [PubMed]
- Giardiello C, Cristiano S, Cerbone MR, et al. Gastric perforation in an obese patient with an intragastric balloon, following previous fundoplication. Obes Surg 2003;13:658-60. [Crossref] [PubMed]
- Genco A, Bruni T, Doldi SB, et al. BioEnterics Intragastric Balloon: The Italian Experience with 2,515 Patients. Obes Surg 2005;15:1161-4. [Crossref] [PubMed]
- Ribeiro da Silva J, Proença L, Rodrigues A, et al. Intragastric Balloon for Obesity Treatment: Safety, Tolerance, and Efficacy. GE Port J Gastroenterol 2018;25:236-42. [Crossref] [PubMed]
- Abou Hussein BM, Khammas AA, Al Ani AM, et al. Gastric Perforation following Intragastric Balloon Insertion: Combined Endoscopic and Laparoscopic Approach for Management: Case Series and Review of Literature. Obes Surg 2016;26:1127-32. [Crossref] [PubMed]
- Muniraj T, Day LW, Teigen LM, et al. AGA Clinical practice guidelines on intra-gastric balloons in the management of obesity. Gastroenterology 2021;160:1799-808. [Crossref] [PubMed]
- Dumonceau JM, François E, Hittelet A, et al. Single vs repeated treatment with the intragastric balloon: a 5-year weight loss study. Obes Surg 2010;20:692-7. [Crossref] [PubMed]
- Neto MG, Silva LB, Grecco E, et al. Brazilian Intragastric Balloon Consensus Statement (BIBC): practical guidelines based on experience of over 40,000 cases. Surg Obes Relat Dis 2018;14:151-9. [Crossref] [PubMed]
- Joffe OY, Molnar IM, Tarasyuk TV, et al. Morphological changes of gastric mucosa after insertion of intragastric balloon. Klin Khir 2015;70-2. [PubMed]
- Atef E, Zalata KR, Atef H, et al. Increased Proliferative Activity Accompanies the Local Inflammatory Response of Gastric Mucosa After Intragastric Balloon Insertion. Dig Dis Sci 2016;61:3498-505. [Crossref] [PubMed]
- Barrichello SA Junior, Ribeiro IB, Fittipaldi-Fernandez RJ, et al. Exclusively endoscopic approach to treating gastric perforation caused by an intragastric balloon: case series and literature review. Endosc Int Open 2018;6:E1322-9. [Crossref] [PubMed]
Cite this article as: Berger ME, Løve US. Gastric perforation during second intragastric balloon treatment: a case report. AME Case Rep 2022;6:15.


