
Bilobectomy for synchronous multiple lung cancer after COVID-19 pneumonia: a case report
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has had a major impact on the medical management of surgical patients. COVID-19 during the perioperative period is a risk factor for increased length of hospital stay, morbidity, and mortality (1). However, there are few studies of elective surgery after COVID-19 pneumonia and it is not known whether previous infection with COVID-19 increases the risk of postoperative complications. Furthermore, histopathological and viral data on resected lung infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are limited. We present a case of successful pulmonary bilobectomy for synchronous triple primary lung cancer in a patient recovering from COVID-19, in accordance with the CARE reporting checklist, and we report the histopathological characteristics of the lung previously infected by SARS-CoV-2. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-21-65/rc).
Case presentation
A 70-year-old woman visited her primary doctor complaining of chest pain. She had a medical history of hypertension, dyslipidemia and hyperuricemia. Chest computed tomography (CT) revealed three abnormal nodules in the right lung: a part-solid ground-glass nodule (GGN) of 4.5 cm in diameter (the solid component was 1.7 cm) in the right upper lobe; a part-solid GGN of 1.8 cm (the solid component was 0.8 cm), and a solid nodule of 0.9 cm in the right middle lobe (Figure 1A,1B). Synchronous triple primary lung cancer was suspected. There were no visible abnormalities that could explain the chest pain.
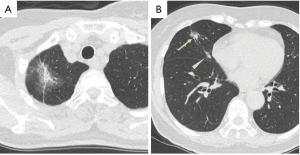
The patient developed a persistent fever before a thorough workup for her lung nodules could be performed. Her chest CT scan revealed newly developed subpleural ground-glass opacities (GGO) in the right lung (Figure 2A). A nasal swab tested positive for SARS-CoV-2 by polymerase chain reaction (PCR) test. The patient was diagnosed with COVID-19 pneumonia and hospitalized. She received combination therapy of favipiravir, camostat mesilate and inhaled ciclesonide (clinical trial: jRCTs031200196) (2). She did not need oxygen supplementation during her hospitalization and was discharged 10 days later. She had no symptoms after the treatment.
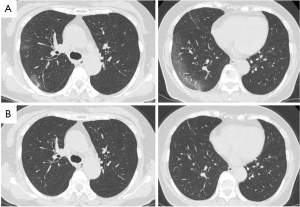
Positron emission tomography scan was performed 14 days after the patient’s COVID-19 diagnosis. Abnormal uptake (a standardized uptake value of 2.7) was observed in the right upper lobe nodule. The right middle lobe nodules showed slight uptake. In addition, lymphadenopathy and abnormal uptake were observed in hilar and mediastinal lymph nodes. Diagnostic bronchoscopy was not performed owing to the potential risk for virus transmission. A CT scan performed 1 month after the COVID-19 diagnosis confirmed shrinkage of the lymph nodes and disappearance of the GGOs (Figure 2B), while the three lung nodules remained persistent. Forced vital cavity (FVC) and forced expiratory volume in 1 second (FEV1) were 2.20 L (91.9% of predicted value) and 1.48 L (85.0%), respectively, and FEV1/FVC was 73.26% 1 month after COVID-19 pneumonia.
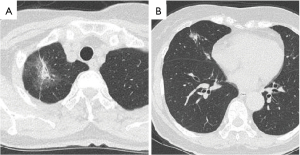
We decided to perform a right upper and middle bilobectomy on this patient to remove the stage IA synchronous triple primary lung cancer. Surgery was scheduled to take place 8 weeks after the patient’s COVID-19 diagnosis in accordance with the recommendations of the American Society of Anesthesiologists (ASA) and the Anesthesia Patient Safety Foundation (APSF) (3). In accordance with our hospital’s policy, a chest CT scan was performed 1 week before surgery, confirming the continued absence of GGOs, and a nasal swab was repeated on the day of admission, which was negative for SARS-CoV-2 by PCR test. There were no changes in the three right lung nodules compared with those of the first visit (Figure 3A,3B).
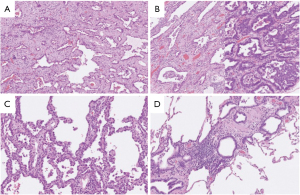
A right upper and middle bilobectomy with regional lymph node dissection was performed via video-assisted thoracoscopic surgery 60 days after the initial COVID-19 diagnosis. There were no adhesions in the thoracic cavity or fibrotic change in the right lower lobe despite the resolved COVID-19 pneumonia. Operative time was 146 minutes and blood loss was 17 mL. The patient’s postoperative course was uneventful and she was discharged 6 days after surgery. Histopathological examination of the nodules revealed adenocarcinoma in situ (AIS) in the right upper lobe (pT1aN0M0, p-stage IA1), invasive adenocarcinoma (lepidic 70%, acinar 30%) (pT1aN0M0, p-stage IA1) in the center of the right middle lobe, and AIS (pTisN0M0, p-stage 0) in the periphery of the right middle lobe (Figure 4A-4C). Histopathological examination of the subpleural right upper and middle lung lobe tissue, where GGOs had been evident due to COVID-19 pneumonia on preoperative CT, showed peribronchial lymphocyte infiltration and interstitial thickening (Figure 4D). Immunohistochemical staining for the SARS-CoV-2 antigen and PCR test for SARS-CoV-2 were both negative in the subpleural lung tissue in which pneumonia had been observed. A CT scan performed 6 months after surgery revealed GGOs continuing absence in the remaining right lower lobe.
All procedures involving a human participant were performed in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of written consent is available for review by the editorial office of this journal.
Discussion
There is limited information on the appropriate timing and management of thoracic surgery after COVID-19 pneumonia; however, the number of such surgical cases is expected to increase in the future. ASA and APSF recommend wait times from the date of COVID-19 diagnosis to surgery as follows: (I) 4 weeks for an asymptomatic patient or a patient with mild, non-respiratory symptoms; (II) 6 weeks for a symptomatic patient who did not require hospitalization; (III) 8 to 10 weeks for a symptomatic patient who is diabetic, immunocompromised, or hospitalized; (IV) 12 weeks for a patient who was admitted to an intensive care unit owing to COVID-19 infection (3). However, a delay in lung resection is independently associated with increased rates of cancer stage progression and decreased median survival times (4). Therefore, the evidence for this recommendation is unclear.
Our patient had been hospitalized and therefore, following the ASA and APSF recommendations, we performed her elective surgery 60 days after her initial COVID-19 diagnosis. However, appropriate timing for elective surgery after COVID-19 pneumonia is still unclear. There are four reported cases of lobectomy to remove lung cancer in patients recovering from COVID-19 (5-7). These are summarized in Table 1. Similar to our case, three cases were categorized in the third group, and one was in the second group according to the ASA and APSF recommendations. The timing of post-COVID surgery varies among the published reports. Lobectomy was performed between 45 days and 16 weeks after an initial COVID-19 diagnosis or 6 weeks after recovery from COVID-19 (5-7). There were no postoperative complications in any reported case. Therefore, we propose that the timing for elective surgical procedure should be determined by taking into account a patient’s general condition, the extent of remaining lung inflammation, and the cancer stage (if applicable). Further studies are required to establish proper guidelines for scheduling elective surgeries after COVID-19.
Table 1
| No. | Author | Age/gender | Category* | Timing | Surgical procedure | Pathological stage |
|---|---|---|---|---|---|---|
| 1 | Testori et al. (5) | 46/male | Third | 45 days from the initial diagnosis | Lobectomy | Stage IIA |
| 2 | Sakai et al. (6) | 65/male | Third | 16 weeks from the initial diagnosis | Lobectomy | Stage IA2 |
| 3 | Nefedov et al. (7) | 65/male | Third | 6 weeks from recovery | Lobectomy | Stage IIIA |
| 4 | Nefedov et al. (7) | 60/male | Second | 6 weeks from recovery | Lobectomy | Stage IA3 |
| 5 | Present case | 70/female | Third | 60 days from the initial diagnosis | Bilobectomy | Stage IA1, IA1, 0 |
*, this category is based on the American Society of Anesthesiologists (ASA) and the Anesthesia Patient Safety Foundation (APSF) proposal.
In our case, SARS-CoV-2 RNA and protein were not detected pathologically in the patient’s lung tissue 60 days after the COVID-19 diagnosis. These results may also suggest that aerosolizing procedures can be safely performed on patients recovering from COVID-19 pneumonia, provided that sufficient intervals are allowed. Therefore, these data could impact the management of patients who have recovered from COVID-19 pneumonia.
In conclusion, we safely performed a bilobectomy for triple primary lung cancer after COVID-19 pneumonia. Further case series are required to establish a safe and appropriate perioperative management system for thoracic surgery in patients recovering from COVID-19 pneumonia.
Acknowledgments
We thank Leah Cannon, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-21-65/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-21-65/coif). Dr. TS reports grants from Japan Agency for Medical Research and Development, during the conduct of the study, grants from Japan Agency for Medical Research and Development, grants from Japan Society for the Promotion of Science, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures involving a human participant were performed in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tian S, Hu W, Niu L, et al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol 2020;15:700-4. [Crossref] [PubMed]
- Japan Primary Registries Network [Internet]. Tokyo: The Association; c2020 [cited 2020 Nov 11]. Available online: https://covid-19.cochrane.org/studies/crs-16897364
- American Society of Anesthesiologists [Internet]. Washington: The Association; c2020 [cited 2020 Dec 8]. Available online: https://www.asahq.org/about-asa/newsroom/news-releases/2020/12/asa-and-apsf-joint-statement-on-elective-surgery-and-anesthesia-for-patients-after-covid-19-infection
- Samson P, Patel A, Garrett T, et al. Effects of Delayed Surgical Resection on Short-Term and Long-Term Outcomes in Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2015;99:1906-12; discussion 1913. [Crossref] [PubMed]
- Testori A, Perroni G, Voulaz E, et al. Pulmonary Lobectomy After COVID-19. Ann Thorac Surg 2021;111:e181-2. [Crossref] [PubMed]
- Sakai T, Azuma Y, Aoki K, et al. Elective lung resection after treatment for COVID-19 pneumonia. Gen Thorac Cardiovasc Surg 2021;69:1159-62. [Crossref] [PubMed]
- Nefedov A, Mortada M, Novitskaya T, et al. Lobectomy with pathological examination in lung cancer patients who recovered from COVID-19. Gen Thorac Cardiovasc Surg 2021;69:1258-60. [Crossref] [PubMed]
Cite this article as: Ishibashi F, Wada H, Kamata T, Terada J, Tsushima K, Hayashi Y, Shiomi T, Takahashi K, Suzuki T, Yoshida S. Bilobectomy for synchronous multiple lung cancer after COVID-19 pneumonia: a case report. AME Case Rep 2022;6:13.


