
Video-assisted thoracoscopic resection of a thoracic inlet schwannoma with neuroforaminal involvement: a case report
Introduction
Neurogenic tumors such as schwannomas are the most common posterior mediastinal mass (1,2). Though benign, their propensity for growth and mass effect on adjacent structures justifies resection (3). Complete resection, rather than enucleation, is key to preventing local recurrence (4). Resection via an approach that combines posterior laminectomy with anterolateral thoracotomy is typically described to improve visualization of key structures including subclavian vessels, the vagus and phrenic nerves, sympathetic chain, and brachial plexus (5,6). We present a patient with a rare subtype of schwannoma of the thoracic inlet involving the T1/2 neural foramen, and which was causing mass effect on the subclavian vein as well as chest wall discomfort. The tumor was entirely resected by video-assisted thoracoscopic surgery (VATS) based on patient preference and with collaboration between thoracic and spine surgery. We additionally detail pre-operative considerations, complications, and suggestions to limit adverse events. The patient enjoyed total symptomatic resolution upon recovery from surgery. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-21-59/rc).
Case presentation
A healthy 57-year-old female was diagnosed with a right-sided T1/2 nerve root schwannoma measuring 6 cm in maximum dimension by fine needle aspiration. The mass was situated in the lung apex and extended into the neural foramen between T1 and T2 causing foraminal widening, as well as mass effect on the right upper lobe and subclavian vein (Figures 1,2). Associated symptoms included chest wall discomfort and shortness of breath, and the patient was noted to have a chronic subclavian vein thrombus likely due to extrinsic compression from this apical lung tumor.
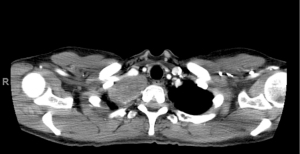
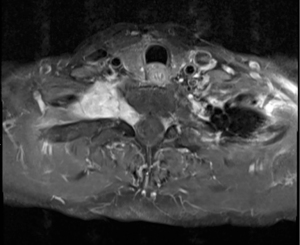
After discussion of risks and benefits, the patient opted for resection via the chest, without exploration of the foraminal component via a posterior laminectomy in order to reduce the chance of T1/T2 nerve root damage and subsequent upper extremity motor complications.
The patient therefore underwent a right-sided video assisted thoracoscopic tumor resection. She was placed laterally, right side up, with arms extended forward. First a camera port was placed in the 7th intercostal space at the anterior axillary line, followed by two additional ports in the 6th intercostal space, one anterior and the other posterior. The pleural overlying the tumor was incised using electrocautery and separated from tumor using blunt dissection and electrocautery. Extra-pleural neovasculature was also divided with electrocautery. The vagus and phrenic nerves were identified and spared. The nerve root stalk which extended towards the neural foramen was completely identified; the extent of the stalk could be visualized with traction of the mass away from midline. The stalk was then transected using the LigaSure device (Medtronic, Minneapolis, MN, USA) and the entire tumor removed from the chest via an endoscopic bag. Permanent pathology confirmed a diagnosis of benign schwannoma. Several days postoperatively the patient experienced worsening positional headache and tinnitus; a commuted tomography myelogram confirmed a dural tear with cerebrospinal fluid leak through the T1/2 foramen, the etiology of which was suspected to be secondary to chronically thinned dura combined with mechanical stress of tumor retraction (Figure 3). In conjunction with a neurosurgeon, the patient returned to the operating room for lumbar drain and dural patch via a right thoracoscopic approach. The same positioning and entry sites as her index surgery were used. Cerebrospinal fluid was noted to be leaking from around the ligated nerve stump. An autologous fat graft from the patient’s lower abdominal wall was placed into the tumor cavity, followed by Evicel (OMRIX Biopharmaceuticals, Ness Ziona, Israel) and dural glue to coat the area. A 4 cm × 5 cm Strattice mesh (Allergan, Dublin, Ireland) was then tacked to the area and an apical pleural tent created to drape over the mesh. Finally, a parietal mechanical pleurodesis was performed down to the level of the diaphragm followed by chemical pleurodesis with talc slurry and doxycycline. After several days of passive drainage of the chest and cerebrospinal fluid, all tubes were removed. The patient thereafter had complete resolution of symptoms and intact upper extremity motor function. Written consent was obtained from the patient to present the information shared here.
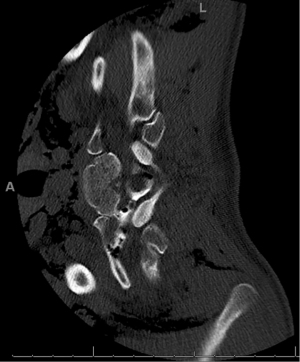
All procedures described here were performed in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
VATS is a viable approach to resection of apical mediastinal tumors. As compared to open surgery or a combined VATS-posterior open approach, VATS involves less blood loss and postoperative pain as well as shorter hospitalization (2). Additionally, the minimally invasive approach does not sacrifice visualization of critical nearby neurovascular structures. In any approach, excellent understanding of thoracic anatomy is essential to avoid intra- and post-operative complications.
Appropriate preoperative evaluation is also key to patient selection and adequacy of resection. Magnetic resonance imaging is valuable for identifying intra-foraminal tumor extension, which may necessitate a combined VATS-posterior laminectomy approach (1,6). Residual tumor in the foramen places the patient at risk for tumor extension into the spinal canal with eventual compression of the spinal cord (3). In this case, minimal foraminal extension enabled complete resection via the chest. Additionally, the tumor must be of a size that can be removed via a VATS port (2). Morcellation of a large tumor should be avoided so as not to spread tumor in the rare event of incorrect diagnosis (2).
Dural integrity should be considered when managing a neural tumor with invasion of the neural foramen. In our case, the avoidance of the neural foramen intraoperatively and the lack of cerebrospinal fluid leak at case conclusion led us not to perform a prophylactic dura repair. However, this may be considered particularly for patients who opt against combined intrathoracic and posterior resection. Additionally, the degree of traction required in this case to expose the length tumor stalk extending towards the neural foramen should have raised the index of suspicion for a possible dural injury and led to prophylactic treatment. There is insufficient data in the thoracic surgery literature to advise one way or another.
In treating this dural injury, given the significant volume of cerebrospinal fluid leakage and symptomatology, we elected for an aggressive transthoracic approach with multimodal pleurodesis to reduce the risk of the patient requiring a morbid complex laminectomy. In the authors’ experience and based on literature review indicating safety of combined modalities for pleural disruption, we elected to use talc, tetracycline, and mechanical pleurodesis (7). The patient suffered no ill effects from the procedure with no effect on pulmonary function. Addition of a fat pad patch, a commonly used technique to protect intrathoracic anastomoses or reinforce esophageal injury repairs, is typically via a pericardial fat pad. However, given a paucity of intrathoracic fat, an abdominal fat pat was used.
As there are few cases of VATS resection of thoracic inlet neurogenic tumors, there is no standardized approach to resection. However, the principles of adequate pre-operative work-up, complete resection of the tumor, and avoidance of damage to nearby structures remain the foundation of successful treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-21-59/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-21-59/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-21-59/coif). Both authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures described here were performed in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kirschbaum A, Ritz R, Pehl A, et al. Giant intrathoracic left-sided vagal schwannoma. Thorac Cardiovasc Surg Rep 2013;2:19-22. [Crossref] [PubMed]
- Kocaturk CI, Sezen CB, Aker C, et al. Surgical approach to posterior mediastinal lesions and long-term outcomes. Asian Cardiovasc Thorac Ann 2017;25:287-91. [Crossref] [PubMed]
- Fierro N, D'ermo G, Di Cola G, et al. Posterior mediastinal schwannoma. Asian Cardiovasc Thorac Ann 2003;11:72-3. [Crossref] [PubMed]
- Etienne H, Agrafiotis AC, Masmoudi H, et al. Postero-apical thoracic schwannoma with cervical extension resected by complete video-assisted thoracoscopic surgery. Monaldi Arch Chest Dis 2019; [Crossref] [PubMed]
- Kayawake H, Chen-Yoshikawa TF, Date H. Dual approach for large mediastinal tumors in the thoracic outlet: transmanubrial osteomuscular sparing approach and video-assisted thoracoscopic surgery. J Cardiothorac Surg 2019;14:42. [Crossref] [PubMed]
- Kim S, Yoo B, Baaj A, et al. Resection of a posterior mediastinal mass: Lessons learned from a failed exploration for presumed schwannoma. J Thorac Cardiovasc Surg 2016;152:e75-e77. [Crossref] [PubMed]
- Hassaan K. Combination therapy with intrapleural doxycycline and talc in reduced doses for pleurodesis. Eur Respir J 2012;40:1265.
Cite this article as: Gologorsky RC, Velotta JB. Video-assisted thoracoscopic resection of a thoracic inlet schwannoma with neuroforaminal involvement: a case report. AME Case Rep 2022;6:12.


