
Aortic valve insufficiency due to myxomatous degeneration: a case report and literature review
Introduction
Aortic valve regurgitation (AVR) is caused by inadequate closure of the valve leaflets. The valve is unable to close tightly after systole allowing blood to regurgitate from the aorta into the left ventricle (1). There are multiple etiologies for AVR. Historically, rheumatic fever was the leading cause of AVR. However, due to the advancement in prophylactic treatment, rheumatic fever is less often the cause in developed countries. Currently, more common causes include endocarditis, aortic dissection, ankylosing spondylitis, congenital valvular defect, connective tissue diseases such a Marfan Syndrome, syphilis aortitis, systemic lupus erythematosus, and trauma, among others (1).
Myxomatous degeneration is derived from the word myxoma. It is described as the “non-inflammatory progressive disarray of the valve structure caused by a defect in the mechanical integrity of the leaflet due to the altered synthesis and remodeling by type VI collagen” (2). The valve leaflets grossly appear thickened with thin and translucent regions in the longitudinal and transversal axis (2). Commonly this process affects not only the valves but also the chordae tendineae. The pathophysiology is not fully understood, but it is thought to be due to an imbalance in the synthesis and degradation of the extracellular matrix (2). This non-inflammatory process most often affects the mitral valve and rarely affects the aortic valve. A common complication of myxomatous degeneration of the aortic valve is the rupture of the chordae tendineae, which leads to acute valvular regurgitation and congestive heart failure. Few cases with variable patient presentations have been reported in the literature. However, no previous literature review has been completed to highlight the various manifestations, diagnostic modalities, and treatment options of myxomatous aortic valve degeneration. Herein, we presented a case of a 64-year-old Caucasian woman with aortic valve insufficiency due to myxomatous degeneration of the aortic valve, which is very uncommon.
We presented the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/acr-21-68).
Case presentation
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
The patient is a 64-year-old Caucasian woman with a past medical history of coronary artery disease, obstructive sleep apnea, chronic kidney disease IIIB secondary to ischemic nephropathy, recurrent deep-vein thromboses (DVTs) (on lifelong apixaban), hypertension, hyperlipidemia, aortic regurgitation, and congestive heart failure who presented to the hospital with shortness of breath, persistent cough, and bilateral lower extremity swelling of one-month duration. The patient noticed a worsening of symptoms over 48 hours prior to presenting to the hospital. The patient denied fever, chills, hemoptysis, or sputum production. The patient had multiple admissions for heart failure in the past year. The vital signs were stable on admission with a temperature of 98.3 degrees Fahrenheit, blood pressure of 134/78 mmHg, heart rate of 94 beats per minute, respiratory rate of 20 breaths per minute, and oxygen saturation of 97%. Pertinent physical exam findings include a significant diastolic murmur on the cardiovascular exam, crackles in the bases of the lungs on the pulmonary exam, and 1+ pitting edema of the bilateral lower extremities. Blood cultures were negative and the comprehensive metabolic panel showed creatinine of 1.82 mg/dL (normal range, 0.6–1.5 mg/dL), BUN 29 mg/dL (normal range, 9.0–27.0 mg/dL), brain natriuretic peptide (BNPEP) 98 pg/mL (normal range, 2–100 pg/mL), and troponin-I high sensitivity 0.0110 (normal range, 0.0000–0.0400 ng/mL). Chest x-ray revealed greater radiodensity of the air space consolidation component of an acute pulmonary process.
A transthoracic echocardiogram (TTE) showed a left ventricular ejection fraction (LVEF) of 50–55%, thickened aortic valve leaflets with moderate AVR and moderate aortic valve stenosis with a peak velocity of 3.9 m/s, mean gradient 37 mmHg, and aortic valve area was 0.99 cm2 (Video 1). The patient was scheduled for a bioprosthetic aortic valve replacement on the following day. The patient underwent aortic valve replacement with 23 mm Inspiris, ligation of the left atrial appendage with a 35 mm AtriClip, and the two aortic valve masses measuring 2.5×1.3×1 and 3×1.5×0.7 cm3 were sent for pathology. The patient was transferred to the coronary care unit (CCU) in stable hemodynamic condition without inotropic support and having received no blood transfusions. After recovery in the CCU, the patient was transferred to inpatient rehabilitation. The pathology report revealed fibrin deposition and fibrous tissue with myxoid degeneration (Figure 1). With continuous improvement, the patient was discharged home on hospitalization day 16. Follow-up outpatient TTE showed LVEF of 55%, and with a normal hemodynamic profile across the bioprosthetic valve without aortic regurgitation or paravalvular leak (Video 2). Table 1 summarizes the patient’s timeline from presentation to discharge and follow-up.
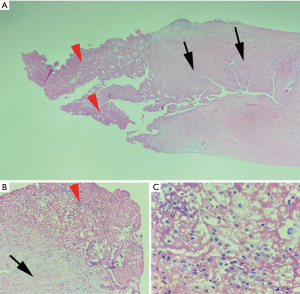
Table 1
| Time | Events |
|---|---|
| One month before admission | The patient started to complain of shortness of breath, persistent cough, and bilateral lower extremity swelling |
| Two days before admission | The patient noticed a worsening of symptoms |
| Hospital day 1 | The patient was admitted to the hospital. The patient had a significant diastolic murmur, crackles in the bases of the lungs, and 1+ pitting edema of the bilateral lower extremities. Chest X-ray revealed greater radiodensity of the air space consolidation component of an acute pulmonary process |
| Hospital day 2 | The TTE showed an LVEF of 50–55%, thickened aortic valve leaflets with moderate aortic valve regurgitation, and moderate aortic valve stenosis with a peak velocity of 3.9 m/s, mean gradient 37 mmHg, and aortic valve area was 0.99 cm2. The patient was scheduled for aortic valve replacement |
| Hospital day 3 | The patient underwent aortic valve replacement and was transferred to the coronary care unit. The pathology report revealed fibrin deposition and fibrous tissue with myxoid degeneration |
| Hospital days 4–7 | The recovery period was uneventful, and the patient was transferred to the inpatient rehabilitation unit |
| Hospital days 8–16 | The patient completed the cardiac rehabilitation and was discharged home |
| Day 30 | The patient had a clinic visit, reported that her symptoms are completely relieved. Follow-up TTE showed LVEF of 55%, and there was a normal hemodynamic profile across the bioprosthetic valve without aortic regurgitation or paravalvular leak |
TTE, transthoracic echocardiogram; LVEF, left ventricular ejection fraction.
Discussion
Myxomatous degeneration commonly involves the mitral valve; however, there are increasing reports of myxomatous degeneration of the aortic valve. This degeneration can lead to clinically significant sequelae resulting in increased associated morbidity and mortality. Myxomatous degeneration is characterized by a non-inflammatory process that resulted in thin and translucent regions macroscopically and disruption of the fibrosa layer due to collagen fiber fragmentation and acid mucopolysaccharide accumulation in the spongiosa microscopically (3). The primary mechanism is unknown. However, a cohort study reported that myxomatous degeneration has a familial dominance mapped to Xq28 gene leading to an imbalance between synthesis and degradation of the extracellular matrix (4).
Aortic valve myxomatous degeneration is becoming an increasingly recognized cause of congestive heart failure exacerbations. Therefore, it is vital to consider it as a differential diagnosis of patients with shortness of breath (1). The most common presentation of myxomatous degeneration of the aortic valve is the sequelae of acute aortic regurgitation, including acute onset or worsening dyspnea, palpitations, orthopnea, chest pain, and peripheral edema (3,5,6), which is a similar course to the patient described above. The severe aortic regurgitation was ultimately associated with a congestive heart failure exacerbation, which can be seen as pulmonary congestion on a chest X-ray (6). TTE is a mainstay in the initial diagnosis of acute onset severe aortic regurgitation. TTE is especially useful in preoperative diagnosis to understand the degree of regurgitation and structural cardiac abnormalities (7).
Common features to be expected on echo in the setting of valvular regurgitation due to myxomatous degeneration include valvular thickening, valvular prolapse, and ruptured chordae tendineae (7,8). The TTE completed on our patient did not show the impact of the myxomatous degeneration on the valvular commissures or coronary cusps. Still, few articles mentioned rupture of fibrous strands at the coronary cusps and avulsion of a commissure due to myxomatous degeneration (6,7). Early diagnosis is crucial to avoid complications associated with myxomatous degeneration of the aortic valve, including acute valvular regurgitation, arrhythmias, congestive heart failure, endocarditis, stroke, and rarely sudden cardiac death (9).
Although medical management is reasonable for acute stabilization and attempts for chronic management, ultimately surgical valve replacement is required, as with the case described above (3,5-7). The valve tissue should be sent to the pathology for diagnostic confirmation and to rule out any malignancy. The patient should be followed up regularly in the clinic until complete recovery.
Few case reports showed patients with various presentations who were later found to have myxomatous aortic valve degeneration leading to their clinical presentation (3,5-8,10,11). Table 2 summarizes the clinical presentations and pathological findings of the cases reported in the literature. Our case is an addition to the above-mentioned cases as described in previous literature to increase physician awareness of myxomatous degeneration of the aortic valve and the importance of considering it as a differential diagnosis in patients presenting with shortness of breath.
Table 2
| Author, year | Patient presentation and pathological findings |
|---|---|
| Uy et al., 1979 (10) | Patient presentation: a 63-year-old male presenting with acute pulmonary edema with a history of dyspnea with exertion, orthopnea, and left ventricular heave with systolic thrill. Grade IV/VI harsh holosystolic murmur at the apex. Presented initially with mitral insufficiency with pulmonary hypertension with subsequent aortic involvement five months post mitral valve replacement. Presentation due to aortic regurgitation included a grade III/VI early diastolic blowing murmur and an II/VI systolic ejection murmur at the left sternal border |
| Pathological findings: large fenestration of the non-coronary cusp with myxoid degeneration of the aortic valve | |
| Hlavaty et al., 1998 (11) | Patient presentation: a total of 56 patients with isolated aortic insufficiency was reported, and all required aortic valve replacement. Around 77% of patients were men, and the mean age was 59 years |
| Pathological findings: around 32% of patients were found to have myxomatous degeneration of the aortic valve only | |
| He et al., 2011 (8) | Patient presentation: 1,080 patients who were undergoing surgery due to moderate to severe cardiac valve regurgitation. Around 68.2% of the patient population were male, and the mean age was 46 years |
| Pathological findings: 104 patients had myxomatous degeneration (9.62%), and 25 of these patients were found to have only aortic involvement, and 10 had both mitral and aortic involvement | |
| Kassem et al., 2014 (3) | Patient presentation: a 69-year-old male presenting with symptoms of acute heart failure, including dyspnea and ankle edema. He has a previous history of atrial fibrillation and mitral valve surgery due to myxomatous degeneration leading to mitral valve prolapse |
| Pathological findings: the patient had myxomatous degeneration of the aortic cusps | |
| Kazuya et al., 2004 (7) | Patient presentation: a 79-year-old male presenting with ascending aortic aneurysm requiring replacement with subsequent acute severe chest pain and symptoms of congestive heart failure. No prior history of heart disease, hypertension, or recent fevers. He had to-and-fro murmur at the left sternal border |
| Pathological findings: the patient had myxomatous degeneration of the aortic valve and medial cystic necrosis of the aorta | |
| Akasaka et al., 2012 (6) | Patient presentation: a 56-year-old female with a history of severe aortic regurgitation presented with worsening dyspnea and palpitations for one month. She had a grade III/IV blowing diastolic murmur at the left sternal border |
| Pathological findings: the patient had myxomatous degenerative changes of the aortic valve with a ruptured fibrous strand attached to the left coronary cusp at the commissure between the left and non-coronary cusps | |
| Akiyama et al., 2004 (5) | Patient presentation: six male patients were reported with a mean age of 53.3 years. Four cases had aortic regurgitation, four patients had 1–2 ruptured fibrous cords at the right coronary cusp, three cases had abnormal fibrous cord attachment to a prolapsing cusp, and one case had an intact fenestrated fibrous cord |
| Pathological findings: all 6 cases had myxomatous aortic valve degeneration, with 3 cases also having myxomatous degeneration of the aortic wall |
Conclusions
Myxomatous degeneration of a cardiac valve has a significant risk of valvular regurgitation. Although it is more commonly associated with the mitral valve, we report the possibility of aortic valve involvement leading to aortic regurgitation. Therefore, it is necessary to include myxomatous degeneration as a possible etiology for aortic regurgitation in patients that do not have other predisposing diagnoses or clinical pictures. Surgical intervention is necessary to consider in the setting of myxomatous degeneration of the aortic valve leading to acute aortic regurgitation with resultant congestive heart failure exacerbation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/acr-21-68
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/acr-21-68). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lindman BR, Bonow RO, Otto CM. Aortic valve disease. In: Zipes DP, Libby P, Bonow RO, et al. editors. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 11th ed. Philadelphia, PA: Elsevier; 2019:chap 68.
- Neto FL, Marques LC, Aiello VD. Myxomatous degeneration of the mitral valve. Autops Case Rep 2018;8:e2018058. [Crossref] [PubMed]
- Kassem S, Polvani G, Al Jaber E, et al. Acute aortic insufficiency due to rupture of an aortic valve commissure. J Card Surg 2014;29:497-8. [Crossref] [PubMed]
- Trochu JN, Kyndt F, Schott JJ, et al. Clinical characteristics of a familial inherited myxomatous valvular dystrophy mapped to Xq28. J Am Coll Cardiol 2000;35:1890-7. [Crossref] [PubMed]
- Akiyama K, Hirota J, Taniyasu N, et al. Pathogenetic significance of myxomatous degeneration in fenestration-related massive aortic regurgitation. Circ J 2004;68:439-43. [Crossref] [PubMed]
- Akasaka K, Saito E, Higuchi T, et al. Aortic regurgitation caused by fibrous strand rupture in a fenestrated aortic valve. J Echocardiogr 2012;10:151-3. [Crossref] [PubMed]
- Kazuya A, Jun H, Naohito T, et al. Echocardiographic and surgical findings of spontaneous avulsion of the aortic valve commissure. Circ J 2004;68:254-6. [Crossref] [PubMed]
- He Y, Guo Y, Li Z, et al. Echocardiographic determination of the prevalence of primary myxomatous degeneration of the cardiac valves. J Am Soc Echocardiogr 2011;24:399-404. [Crossref] [PubMed]
- Bekeredjian R, Grayburn PA. Valvular heart disease: aortic regurgitation. Circulation 2005;112:125-34. [Crossref] [PubMed]
- Uy SA Jr, Taylor PC, Kramer JR. Case report: progressive myxomatous degeneration of the cardiac valves. Cleve Clin Q 1979;46:23-7. [Crossref] [PubMed]
- Hlavaty L, Heide RV. Causes of isolated aortic insufficiency in an urban population in the 1990s a review of 56 surgical pathology cases. Cardiovasc Pathol 1998;7:313-9. [Crossref] [PubMed]
Cite this article as: Abdelazeem B, Hollander RM, Gresham TM, Gjeka R, Kunadi A. Aortic valve insufficiency due to myxomatous degeneration: a case report and literature review. AME Case Rep 2022;6:10.


