
NUT midline lung cancer: a rare case report with literature review
Introduction
Nuclear carcinoma of the testis (NUT) are rare squamous cell neoplasms that can occur at multiple organ sites and are characterized by an aggressive clinical course. This tumor is known to occur in the midline structures, with predominance in the head and neck, and mediastinum (1). NUT carcinoma usually presents at an advanced stage with rapid progression to death. Data on prognosis and survival statistics is very limited for these patients due to the low incidence of this malignancy. Despite intensive treatments, only 20 percent of patients survive one year (2). Additional research and clinical trials are needed to fully understand the characteristics of these patients and the efficacy of various treatment regimens.
NUT carcinomas are poorly differentiated and are genetically defined by the presence of a rearrangement of the NUT M1 gene, resulting in fusion of NUT to the bromodomain family members BRD3 or BRD4, or the methyltransferase NSD3, and leads to epigenetic reprogramming and resultant loss of cell differentiation. The chromosomal translocation occurs between NUTM1 located in the chromosome 15q14 and either BRD3 in chromosome 9q34.2 (6%), BRD4 in chromosome 19p13.1 (70%), or another/unknown gene (24%). The tumor has histopathologic similarity with squamous cell cancer therefore may not be easy to identify. If there is suspicion of NUT then immunohistochemical (IHC) stain is used to confirm the diagnosis. IHC uses monoclonal antibody to NUT (C52, Cell Signaling) which has been shown to be 87% sensitive and 100% specific. Diagnosis can also be made by molecular analysis showing NUT rearrangement, including fluorescent in situ hybridization (FISH), reverse transcriptase-polymerase chain reaction (RT-PCR), or cytogenetic analysis (2).
We present the following article in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/acr-21-35).
Case presentation
A 49-year-old-male with no past medical history presented with cough that worsened over the past two weeks prior to his initial presentation, becoming persistent and productive of yellow sputum with occasional hemoptysis. This was associated with intermittent right-sided chest pain, but he denied shortness of breath, fever, and weight loss. No history of cigarette smoking. There were no significant abnormal findings on physical exam.
A chest X-ray 4 days prior to presentation showed a well-circumscribed lobulated mass within the posteromedial right upper lobe of the lung (Figure 1A). A computed tomography (CT) scan (Figure 1B) performed a few days later further characterized the opacity as a 6.6×6.0×5.9 cm3 solid, heterogeneous mass in the superior right upper lobe, obliterating the right upper bronchus and abutting the pleura. Additionally, two solid 4 mm left upper lobe pulmonary nodules, a 5.3×4.1 cm2 paratracheal lymph node, and generalized mediastinal lymphadenopathy were demonstrated.
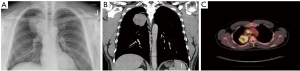
The patient then had a mediastinoscopy with paratracheal lymph node biopsy that demonstrated cells with large nuclei, prominent nucleoli, and fairly scant cytoplasm. The tumor possessed a solid architecture with areas of discohesion and necrosis. A panel of IHC markers were negative, except for positive trace TTF-1 and cytokeratin (negative for Claudin-4, Napsin, Synaptophysin, Chromogranin, CD56, p40, WT-1, CD3, CD20, SOX10, CD34, ERG, and OCT3/4). The architecture and IHC markers led to a tentative diagnosis of poorly differentiated adenocarcinoma of the lung. A positron emission tomography (PET) scan showed increased FDG uptake in the right upper lobe mass as well as in the right paratracheal and suprahilar region with no sites of increased uptake outside of the thorax (Figure 1C), consistent with the preceding CT scan. A brain MRI revealed no metastatic disease. Next-generation sequencing revealed the presence of a NUTM1-BRD4 fusion, identifying the mass as a NUT carcinoma and the patient was diagnosed with NUT midline lung cancer, clinical stage IIIB.
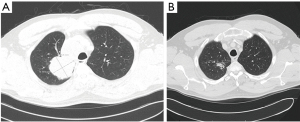
The tumor was deemed unresectable, and the decision was made to begin definitive chemoradiation. The patient received 7 weeks of weekly carboplatin and paclitaxel ending on DAP 72. The patient also received a total of 6,000 cGy of radiation, the whole right lung received 13 fractions of 200 cGy of photons while the right upper lobe and mediastinum received 17 fractions of 200 CcGE of protons. A CT scan five weeks following completion of the chemoradiation therapy showed partial response (PR) (Figure 2). The patient was started on consolidation therapy with durvalumab.
Months 6–9: extra-thoracic spread
Six months after the initial presentation the patient was seen by an orthopedic surgeon for the development of a palpable lump in his left arm along the lateral triceps with associated numbness on dorsal forearm and hand weakness. An MRI of the humerus demonstrated a large, lobulated, infiltrative enhancing mass within the posterior muscle compartment. A biopsy taken from this mass confirmed NUT carcinoma of lung based on the molecular testing revealing NUTM1-BRD4 fusion.
At this point, the patient underwent restaging with a brain MRI and PET CT, neither of which demonstrated evidence of metastatic disease. The patient received 6,000 cGy of radiation divided into 30 fractions of 200 cGy of photons directed at the triceps lesion. The follow up scans showed complete response (CR).
Months 10–13: thoracic reoccurrence
A CT scan showed interval development of several new nodules in the lungs bilaterally triggering additional restaging with a humeral MRI on the same day and a PET scan and brain MRI, all of which were remarkable only for post-radiation changes. Compassionate-use molibresib 120 mg daily (GSK 525762) was started. Follow up CT scan 6 weeks later showed an interval increase in size and number of presumed pulmonary metastatic foci with new pathologic subcarinal and left hilar adenopathy, consistent with disease progression. Four weeks later, the molibresib dose was reduced to 60 mg per day for fatigue, decreased appetite, and thrombocytopenia.
Month 14: intracranial spread and intra-thoracic disease progression
About 30 days later, the patient presented to the emergency department with severe headache and confusion following an intense episode of coughing; he was found to have a brain metastasis with resultant left parieto-occipital hemorrhage causing subfalcine and uncal herniation (Figure 3A), for which he had a craniotomy with tumor resection. Surgical pathology results were consistent with metastatic NUT carcinoma both by IHC and molecular testing. He underwent gamma knife radiosurgery to the solitary metastasis with 3,000 cGy delivered in 5 fractions, achieving CR.
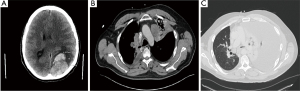
CT chest/abdomen and pelvis (Figure 3B), and during this admission showed significant progression of pulmonary nodular and nodal metastasis in the thorax with interval post obstructive atelectasis/consolidation of the left upper lobe. No metastatic disease was found in the abdomen and pelvis.
Month 15: complications and subsequent treatments
Due to worsening respiratory status and disease progression as demonstrated on chest CT, the patient underwent radiation to the left lung and hilum; a total of 10 fractions of radiation were delivered with the first being an AP/PA plan using 15 MV photons to deliver 300 cGy and the remaining nine were via IMRT, using 18 MV photons to deliver 2,700 cGy, achieving PR with some relief in respiratory symptoms.
Months 15–16: initiation of chemo-immunotherapy for progressive disease
Carboplatin-pemetrexed-pembrolizumab was initiated with neulasta. The patient received two cycles of this treatment but was admitted to the hospital for fever, shortness of breath, and a productive cough. A chest CT (Figure 3C) demonstrated a large left pleural effusion with worsening metastatic pulmonary disease. The following day, the patient underwent a thoracentesis for the pleural effusion; however, it quickly reaccumulated requiring placement of a pleurx catheter and discharged home with skilled home care. Unfortunately, the patient passed away a month later from worsening respiratory distress.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Patient consent was not included as we have not disclosed any patient identifiers.
Discussion
NUT midline carcinoma (NMC) is an extremely rare, aggressive type of squamous cell neoplasm that portends poor prognosis. The exact incidence and prevalence of this malignancy is difficult to estimate due to its rarity. It is important to have high clinical suspicion for this diagnosis in young patients initially found to have undifferentiated or poorly differentiated tumors involving the midline structures since NMC arises from thoracic cavity structures in almost half of the cases, followed by head and neck (1,2). Our patient was a 49-year-old male initially diagnosed with poorly differentiated adenocarcinoma of the lung based on architecture and IHC markers of the biopsy taken from the right paratracheal lymph node. Further testing three weeks later with next-generation sequencing revealed the presence of a NUTM1-BRD4 fusion confirming the diagnosis of NMC. NUTM1-BRD4 fusion is the most common genetic translocation found in NMC.
In a relatively large study, Chau et al. assessed risk factors associated with worse clinical outcome in 124 patients with diagnosis of NMC. Median age at diagnosis was 23.6 years (1). The results showed statistically significant associations between prognosis and type of translocation (P=0.024), attributing worse prognosis to the translocation of NUTM1 gene to BRD4 compared to BRD3, or NSD3. Furthermore, the primary location of the tumor was also noted to have a prognostic impact as well, favoring patients with an extra-thoracic primary tumor compared to primary thoracic involvement (P<0.0001). Other risk factors associated with worse outcomes included lymph node and organ involvement at initial presentation, and tumor size ≥6 cm. Patients with primary thoracic mass and NUTM1-BRD4 were found to have a median overall survival of 4.4 months, compared to 36.5 overall survival for patients with a non-BRD-4, extra-thoracic primary (1). Our patient with a primary thoracic tumor, a NUT-BDR4 translocation and a mass size >6 cm was expected to have poor prognosis, as his initial diagnosis was classified as a stage IIIB NUT carcinoma. No predisposing factors have been identified for this malignancy. In a small case series including eight NMC cases with primary lung tumor, 75% of patients had minimal or no significant smoking history, and all of whom had a centrally located unilateral pulmonary mass, as did our patient (3).
Unfortunately, a standard therapy regimen has not yet been developed for this rare aggressive tumor. Treatment choices generally start with surgical resection, followed by adjuvant radiotherapy or combined chemoradiation. A small cohort study that included 63 NMC patients showed 80% 2-year overall survival in patients who received complete resection (P=0.002) (2). However, because our patient’s primary tumor was deemed unresectable, the decision was made to initiate therapy with concurrent chemoradiation. The choice of systemic therapy regimens include platinum, anthracyclines, and alkylating agents used in combination. The primary response to therapy is variable; however, primary tumors are often sensitive to radiotherapy. Small studies have also shown that patients who initially received radiotherapy had a better 2-year and progression-free survival (2). The primary tumor in this case showed significant response to concurrent chemoradiation with seven cycles of carboplatin and paclitaxel with radiotherapy.
The most common treatment approaches reported in the literature were based on pathological features and IHC staining, which in the majority of the cases were initially misdiagnosed as poorly differentiated non-small cell lung cancer, melanoma, or germ cell tumor (3,4). Our patient’s initial histopathology was consistent with poorly differentiated adenocarcinoma. We decided to treat as a non-resectable stage IIIB non-small cell lung cancer and prescribed a consolidation therapy with the immune checkpoint inhibitor durvalumab for almost 2 months after initial positive response with concurrent chemoradiation. During this time our patient experienced partial response, which has not been previously reported with this combination in NUT carcinoma.
Consistent with the known aggressive malignancy of NUT, we sadly were not able to prevent the rapid progression of the disease. Our patient developed metastases within 2 months of durvalumab treatment, which was discontinued due to progressive disease. Metastatic sites included the muscular compartment of the humerus, which eventually responded to six weeks of radiotherapy.
Both bromodomain and extra-terminal Motif (BET) and histone deacetylase (HDAC) inhibitors have the potential for efficacy against NMC by inducing cellular differentiation and may possibly impact survival. Results of phase I clinical trials have shown partial efficacy of agents directly targeting BRD3 and BRD4 with 20–30% partial response. Our patient received a BET inhibitor for a period of two months, but he could not tolerate the initial recommended dose and his disease continued to progress. During the last few months, his disease metastasized to the brain, with progressive pulmonary nodule and mediastinal lymphadenopathy.
NMC remains challenging to treat and has a generally low estimated median overall survival ranging from 6.7 to 9.7 months (2,5). In this case, we were able to achieve a relatively longer survival than expected, as our patient survived 18 months after initial diagnosis.
Screening for NUT protein rearrangement should be considered in poorly differentiated neoplasms arising from midline structures, as early identification of NMC and enrollment in clinical trials could possibly reveal superior therapeutic options and potentially improve survival and clinical outcomes.
Conclusions
NMCs are one of the rare and extremely aggressive squamous cell neoplasms, which predominantly arise in the thorax, head and neck and carry a dismal prognosis. Initial surgical resection seems to be significantly associated with better progression free and overall survival. For patients who are not surgical candidates, we do not have effective treatments. Clinical trials have shown early success with molecularly targeted therapies; therefore, patients should be encouraged to enroll in clinical trials of these promising therapeutic strategies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/acr-21-35
Peer Review File: Available at https://dx.doi.org/10.21037/acr-21-35
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/acr-21-35). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Patient consent was not included as we have not disclosed any patient identifiers.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chau NG, Ma C, Danga K, et al. An anatomical site and genetic-based prognostic model for patients with nuclear protein in testis (NUT) midline carcinoma: analysis of 124 patients. JNCI Cancer Spectrum 2019;4:pkz094. [Crossref] [PubMed]
- Bauer DE, Mitchell CM, Strait KM, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res 2012;18:5773-9. [Crossref] [PubMed]
- Sholl LM, Nishino M, Pokharel S, et al. Primary Pulmonary NUT Midline Carcinoma: Clinical, Radiographic, and Pathologic Characterizations. J Thorac Oncol 2015;10:951-9. [Crossref] [PubMed]
- Elkhatib SK, Neilsen BK, Sleightholm RL, et al. A 47-year-old woman with nuclear protein in testis midline carcinoma masquerading as a sinus infection: a case report and review of the literature. J Med Case Rep 2019;13:57. [Crossref] [PubMed]
- Chau NG, Hurwitz S, Mitchell CM, et al. Intensive treatment and survival outcomes in NUT midline carcinoma of the head and neck. Cancer 2016;122:3632-40. [Crossref] [PubMed]
Cite this article as: Gupta R, Mumaw D, Antonios B, Anusim N, Dhulipalla SP, Stender M, Huben M, Jaiyesimi I. NUT midline lung cancer: a rare case report with literature review. AME Case Rep 2022;6:2.


