
Pleomorphic adenoma of the maxillary sinus with orbital extension presenting with exophthalmos: a case report
Introduction
Pleomorphic adenomas (PA) of the nasal area and paranasal sinuses are extremely rare and slowly progressive benign tumors. PAs are mostly encountered in the major salivary glands, typically in the parotid, comprising 80% of all benign parotid neoplasms, and less frequently in the minor salivary glands of the soft palate, nasal cavity and paranasal sinuses, as well as the nasopharynx, lacrimal glands, trachea and larynx (1). Herein, we report a rare case of PA located in the maxillary sinus, with an unusual facial presentation, accentuating that the unusual site of the mass could preclude its timely diagnosis and be associated with complications. The diagnostic and therapeutic approach selected for our patient are discussed, in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/acr-21-31).
Case presentation
A 66-year-old Caucasian male presented to the Emergency ENT Department of the University Hospital of Patras, complaining of progressively worsening symptoms of nasal obstruction on the left side. The patient had been experiencing nasal congestion and blockage for the past year, while he reported deterioration in nasal breathing and excessive snoring during the last 6 months prior to his presentation. A slowly developing proptosis of the left eye was also observed by the patient over the past 4 months, without any other ocular symptoms. He reported no rhinorrhea, episodes of epistaxis or allergy history. His past medical history included head injury due to a motor vehicle accident 20 years ago, while the remainder of his medical history was otherwise unremarkable.
The patient was admitted for further investigation in the ENT department. On clinical examination, there was marked proptosis of the left globe, while anterior rhinoscopy and fiberoptic nasendoscopy revealed a mass located in the left nasal cavity blocking the left nostril. Furthermore, a left deviation of the nasal septum and oedema of the right inferior nasal turbinate were noticed. Intraoral and ear examination was normal. Ophthalmic evaluation showed subtle restriction in the downward gaze with no other defects in ocular motility, while visual acuity and fundus examination were normal.
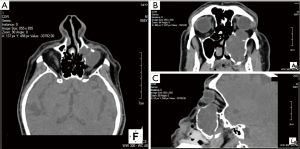
High resolution computed tomography (CT) scan of the paranasal sinuses demonstrated complete occupation of the left maxillary sinus by soft tissue elements with extension to the adjacent structures. CT findings included erosion of the medial wall of the maxillary sinus and extension of the mass into the nasal cavity up to the nasal septum causing obstruction of the airway. Furthermore, extension of the mass to the orbital floor coming in contact with the left globe resulted in pressure to the left periorbital area, with mild proptosis and possibly erosion of the left inferior rectus muscle (Figure 1). Based on the features described in CT, a mucocele was set high in the differential diagnosis.
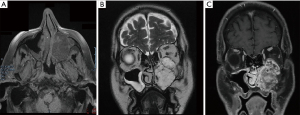
For further evaluation of the soft tissue component of the mass, magnetic resonance imaging (MRI) was performed. MRI confirmed the complete occupation of the left maxillary antrum by material characterized by heterogenicity and intermediate signal intensity in T1-weighted MR images and high signal intensity in T2-weighted MR images (Figure 2A). After intravenous injection of paramagnetic contrast agent, signal intensity increase (enhancement) of the mass was observed (Figure 2B). Based on the unilateral nature of the disease and its radiographic features, the differential diagnosis included an inverted papilloma, lymphoma, squamous cell carcinoma, adenomatous tumors such as the cystic adenocarcinoma, or another neoplasm arising from the maxillary sinus.
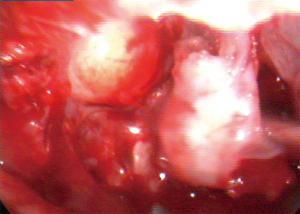
Due to the extent and location of the lesion, accessing the mass endoscopically in order to perform a preoperative biopsy was not possible. Therefore, for both diagnostic and therapeutic purposes, functional endoscopic sinus surgery (FESS) under general anesthesia with the surgical plan of an extended biopsy was considered the treatment of choice. Surgery included endoscopic septoplasty for the deviated nasal septum. Following that, a medial maxillectomy was performed. Intraoperatively, a soft tissue tumor, attached to the lateral wall of the maxillary antrum expanding to the orbit with attachment to the periorbita was observed (Figure 3). En bloc resection of the mass was achieved with clear resection/surgical margins after thorough dissection and separation from the surrounding tissues. The tissue removed was sent for pathology examination.
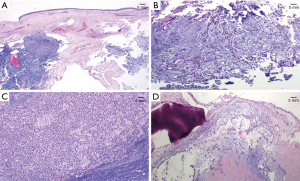
Histopathology report revealed a biphasic tumor, developing under the pseudostratified columnar epithelium, composing of both epithelial and stromal component with moderate cellularity. The two elements were closely mixed. The epithelial cells were forming tubules, sheets with ductular structures and cords, while the stromal component had myxoid and focally cartilaginous differentiation. Immunohistochemical stains were performed in order to confirm the biphasic elements (Figure 4A-4D). Epithelial cells were positive for p63 and negative for S100 while the stromal cells were positive for S100 and negative for p63. Based on the morphology and the immunohistochemical stains, the diagnosis was mixed tumor/PA of the maxillary sinus surrounded by respiratory mucosa without any evidence of malignancy. Resection margins were clear. The post-operative period was uneventful. Left exophthalmos resolved after surgery. Short- and long-term prognosis following complete tumor excision was considered excellent. However, due to the small risk of recurrence the patient remained under close follow up every 3 months during the first year, with imaging and repeated nasal endoscopic examination without any signs of recurrence.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
PA is the most common benign salivary gland tumor, accounting for 45–74% of all salivary gland neoplasms. It typically arises from major salivary glands, namely the parotid in 65% of the cases (2). PAs constitute mixed tumors consisting of epithelial, myoepithelial and stromal component with well demarcated margins separated from healthy tissue by a fibrous capsule. Epithelial differentiation as well as the diversity of the stromal patterns are responsible for its high pleomorphism (3). More specifically, the epithelium can form sheets or duct-like structures. Myoepithelial cells may be arranged either in a reticular pattern or sheets of spindle shaped cells, while their stromal component can be myxoid, fibrous/hyalinized or chondroid.
About 10% of all PAs originate from minor salivary glands distributed throughout the upper aerodigestive tract. PAs of the upper respiratory airway are rarely described and usually affect the nasal cavity with involvement of the septum in 82.5–90% of the reported cases, followed by the maxillary sinus and nasopharynx (4,5). They usually present between 30 and 60 years of age with a slight predilection for women. As opposed to major salivary glands’ PAs which are characterized by relatively low myoepithelial cellularity, the ones of the aerodigestive tract exhibit high cellularity and absence of stromal component, simulating more aggressive epithelial neoplasms.
The origin of sino-nasal PAs remains debatable. A theory proposed from Matthew et al. suggests that a misplaced embryonic epithelial cell in the mucosa of nasal septum could be the cause of a PA (6). Another theory supports that these PAs may originate from remnants of the vomeronasal organ from early fetal life (7).
Although PAs are benign, local recurrence is a potential risk, while malignant transformation into carcinoma ex-PA is encountered in about 6% of all PA cases (8). Following resection, the recurrence rates reported vary from 0 to 8%, while multiple recurrences are associated with malignant transformation (9). The presence of a highly myxoid stroma and an irregular or invaded capsule with multinodularity increase the risk of local recurrence. Metastasis can also occur particularly in cases where enucleation or incomplete excision is performed.
Clinical presentation is with nasal obstruction (in 71% of cases), epistaxis (56%), epiphora, rhinorrhea. Occasionally, an external deformity of the nose may be observed depending on the bulk of the tumor. Imaging with both CT and MRI is of paramount importance when clinical suspicion of a neoplasm is present.
Diagnostically, an extended biopsy with debulking through an endonasal, endoscopic approach when easily accessible, may be optimum for establishing a correct diagnosis especially in cases of large tumors, all the while providing patient relief from symptoms of obstruction. Wide surgical excision is the mainstay of treatment aiming for clear clinical margins of at least one centimeter to avoid possible recurrence. Postoperative radiotherapy has been suggested by some authors in the presence of residual disease (4).
As far as the PAs of the maxillary sinus are concerned, there are few case reports in literature. A review of 40 cases of intranasal PAs from Compagno and Wong, reported 23 cases in females and 17 in males, 62% of which originated in the nasal septum. No cases primarily involving the maxillary sinus were found. However, three cases showed extension of the PA from the nasal septum to the maxillary sinus (10). In a review of 2,807 cases of salivary gland tumors, Spiro reported no tumors located in the maxillary and ethmoidal sinuses (11). Similarly, in a retrospective analysis of benign sinonasal tumors managed with FESS, 2 out of 105 cases were PAs, indicating the rarity of the tumor in the specific location (12).
Martis and Karakasis were the first to report a case of maxillary sinus PA presenting with cheek swelling in 1971 (13). Cases presenting with similar symptoms have been described by Leunig involving an elderly patient complaining of nasal obstruction and occasional epistaxis (14) and Berenholz et al. (15). Another case showing repeated recurrence and bone formation was reported by Lee et al. (5). All of the abovementioned cases were managed with open surgery. In 2002, Facon et al. described a case arising from the medial wall of the maxillary antrum treated via an endoscopic approach (16). Ray et al. reported a case of a large PA of the maxillary antrum with extensive ossification presenting with maxillary swelling mistakenly attributed to odontogenic origin (17). An intra-sinus PA with comparable clinical presentation in a 17-year-old was reported by Razafindrakoto et al. (18). Lately, a retrospective analysis of 17 patient’s data also included one case of PA originating from the maxillary sinus (19). According to a case series presentation based on retrospective review by Kuan et al. including 39 patients endoscopic resection was employed in 66% of cases, which achieved oncologic control in more than 80% of cases at a mean follow-up of 2 years (20). Although no cases of PA were reported, one case of carcinoma ex-PA originating from the maxillary sinus was found, as PAs are capable of undergoing malignant transformation.
We report an atypical case of unilateral PA originating from the maxillary sinus, presenting with proptosis due to erosion of the orbital floor and invasion of the orbit by the tumor. Taking into consideration the radiographic studies, we proceeded surgically with an extended surgical biopsy, which set the diagnosis of PA. Endoscopic sinus surgery ensured local but adequate tumor resection with no recurrence at 1 year follow up in our patient. However, a limitation that applies in our case is the lack of long-term follow-up and empirical surveillance data availability, as PA recurrence tends to occur later than 2 years post-resection.
To conclude, except for the rarity of the disease, this case highlights that high clinical suspicion is required in cases of unilateral nasal obstruction, particularly when accompanied by orbital features. Given the nature and chronicity of symptoms, early detection of such pathology necessitates proper investigation with endoscopy and imaging. In the absence of clinically evident characteristic findings of sino-nasal PAs, we emphasize the role of imaging with CT and MRI in evaluating unilateral invasive lesions of the area. Additionally, accurate tumor sampling is vital for prompt diagnosis and management. It is worth mentioning that the majority of patients may be eligible for endoscopic surgical excision, providing a satisfactory treatment outcome without radical procedures. Long-term follow-up is necessary for monitoring possible recurrence of the tumor.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/acr-21-31
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/acr-21-31). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Eneroth CM. Salivary gland tumors in the parotid gland, submandibular gland, and the palate region. Cancer 1971;27:1415-8. [Crossref] [PubMed]
- Ellis GL, Auclair PL. editors. Atlas of tumor pathology. Tumours of the salivary glands. Washington, DC: Armed Forces Institute of Pathology, 1995:39-41.
- Evasion JW, Kusufuka H, Stenman G, et al. Pleomorphic adenoma. In: Barnes L, Eveson JM, Reichard P, Sidransky D. editors. Pathology and genetics, head and neck tumours, World Health Organization classification of tumours. Lyon: IARC Press, 2005:254-8.
- Mackle T, Zahirovic A, Walsh M. Pleomorphic adenoma of the nasal septum. Ann Otol Rhinol Laryngol 2004;113:210-1. [Crossref] [PubMed]
- Lee KC, Chan JK, Chong YW. Ossifying pleomorphic adenoma of the maxillary antrum. J Laryngol Otol 1992;106:50-2. [Crossref] [PubMed]
- Matthew S, Ersner MD, Saltzman M. A mixed tumor of the nasal septum: report of a case. Laryngoscope 1944;54:287-96.
- Stevenson HN. Mixed tumor of the septum. Ann Otol Rhinol Laryngol 1932;41:563-70. [Crossref]
- Cimino-Mathews A, Lin BM, Chang SS, et al. Carcinoma ex pleomorphic adenoma of the nasal cavity. Head Neck Pathol 2011;5:405-9. [Crossref] [PubMed]
- Suh MW, Hah JH, Kwon SK, et al. Clinical manifestations of recurrent parotid pleomorphic adenoma. Clin Exp Otorhinolaryngol 2009;2:193-7. [Crossref] [PubMed]
- Compagno J, Wong RT. Intranasal mixed tumors (pleomorphic adenomas): a clinicopathologic study of 40 cases. Am J Clin Pathol 1977;68:213-8. [Crossref] [PubMed]
- Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg 1986;8:177-84. [Crossref] [PubMed]
- Baradaranfar MH, Dabirmoghaddam P. Endoscopic endonasal surgery for resection of benign sinonasal tumors: experience with 105 patients. Arch Iran Med 2006;9:244-9. [PubMed]
- Martis CS, Karakasis DT. Pleomorphic adenoma arising in the maxillary sinus. Case report. Plast Reconstr Surg 1971;47:290-2. [Crossref] [PubMed]
- Leunig A, Grevers G. Pleomorphic adenoma of the maxillary sinus. Laryngorhinootologie 1994;73:595-6. [Crossref] [PubMed]
- Berenholz L, Kessler A, Segal S. Massive pleomorphic adenoma of the maxillary sinus. A case report. Int J Oral Maxillofac Surg 1998;27:372-3. [Crossref] [PubMed]
- Facon F, Paris J, Ayache S, et al. Pleomorphic adenoma of the nasal cavity: a case arising from the wall of the maxillary sinus. Rev Laryngol Otol Rhinol (Bord) 2002;123:103-7. [PubMed]
- Ray D, Mazumder D, Ray J, et al. Massive ossifying pleomorphic adenoma of the maxillary antrum: a rare presentation. Contemp Clin Dent 2015;6:139-41. [Crossref] [PubMed]
- Razafindrakoto RM, Schammirah MR. Intra-sinusal maxillary pleomorphic adenoma: a rare entity. Pan Afr Med J 2015;22:81. [Crossref] [PubMed]
- Li W, Lu H, Zhang H, et al. Sinonasal/nasopharyngeal pleomorphic adenoma and carcinoma ex pleomorphic adenoma: a report of 17 surgical cases combined with a literature review. Cancer Manag Res 2019;11:5545-55. [Crossref] [PubMed]
- Kuan EC, Diaz MF, Chiu AG, et al. Sinonasal and skull base pleomorphic adenoma: a case series and literature review. Int Forum Allergy Rhinol 2015;5:460-8. [Crossref] [PubMed]
Cite this article as: Lygeros S, Tsapardoni F, Mastronikolis S, Axioti AM, Grypari IM, Danielides G, Naxakis S. Pleomorphic adenoma of the maxillary sinus with orbital extension presenting with exophthalmos: a case report. AME Case Rep 2021;5:39.


