Chiropractic care for low back pain, gait and posture in a patient with Parkinson’s disease: a case report and brief review
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder that affects mainly the dopaminergic neurons in the substantia nigra pars compacta (1). PD is classified as an extrapyramidal and movement disorder in the ICD-10-CM (G20-26) (2). Pain is a common non-motor symptom in PD that markedly impacts patients’ quality of life, with prevalence from 40% to 75% of those with PD (3). PD pathology is thought to have a modulating effect on pain sensation, which could amplify pain (4). Patients with motor symptoms such as rigidity, gait difficulty, and postural instability have a higher frequency and severity of painful symptoms (5,6), while those with solely tremor symptoms is not associated with pain (5). It is speculated that patients with motor symptoms have difficulties in executing repetitive movements and performing daily tasks, and repeated efforts to perform tasks may trigger pain (5,6).
There is no cure for PD itself, but medications can offer benefits in terms of controlling the motor and painful symptoms (7). In treatment of PD-related pain, dopaminergic agents, analgesics, antidepressants and physical therapies are commonly employed (8). Aging changes in the muscles, bones and joints may accompany PD and also impact performance at work or of daily tasks. Functional capacity of the musculoskeletal system can be enhanced by different types of manual therapy, by means of improving muscle strength, joint mobility, and postural balance. Presented is a case of PD with pre-existing low back pain. While the musculoskeletal pain was resolved with chiropractic intervention, stable dynamic gait, better postural alignment and stability were achieved concurrently as well. The current study may serve as an example of chiropractic manipulation showing the potential to address gait and posture problems associated with pain in a patient with PD. Non-pharmacological interventions are often required for a specific indication when these would benefit the individual patient. The potential contribution of manipulative treatments in PD should not be overlooked.
We present the following article in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/acr-21-27).
Case presentation
A 59-year-old Chinese male taxi-driver presented with progressive lower back pain radiating to the right leg and walking difficulty for 2 months. The patient reported a three-year history of work-related back pain. Pain was usually minimal (average intensity 2/10 on an 11-point numeric pain rating scale), but aggravated (intensity 4/10) by driving for a long time or lifting heavy objects. He had PD diagnosed 18 months ago with and initially responded to levodopa. After 1 year on levodopa, he felt progressively worsening stiffness of the legs, difficulty in initiating walking, forward-stooped posture, and balance problems. He had sought treatment from an orthopedic surgeon. Brain magnetic resonance imaging (MRI) and extracranial magnetic resonance angiography excluded stroke or cerebral pathologies. Spine MRI showed cervical and lumbar spondylosis, intervertebral disc prolapses from L1/L2 to L5/S1 levels with compression of bilateral S1 nerve roots, compatible with S1 radiculopathy caused by lumbar disc herniation. The patient had tried physiotherapy but stopped engaging it due to unbearable pain during the lumbar traction procedure. His current oral medication included levodopa and non-steroid anti-inflammatory drugs, although both did not properly control his motor symptoms or pain. Over the past 2 months, his back pain and walking difficulty got worse with comorbid insomnia and depressed mood. The patient rated the maximum intensity of his back pain as 8 out of 10 on a numeric rating scale. He could not continue driving taxi and making daily tasks.
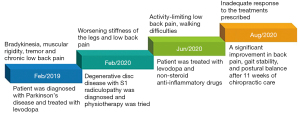
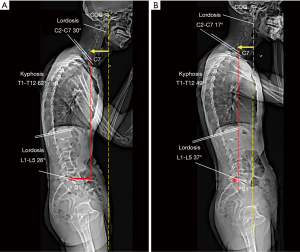
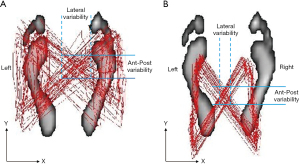
At presentation, he showed bradykinesia and difficulty walking. Examination revealed features of stiffness in bilateral cervical paraspinal, upper trapezius and scalene muscles, tenderness and spasms of the right L5–S1 paraspinal muscles and diminished motor strength in right hip flexion and knee extension (grade 4/5). Standing EOS® radiographs (Figure 1A) revealed characteristic features of PD, including forward head, stooped posture, straightening of the lumbar spine, slightly flexed hips and knees, and anterior deviation of the center of gravity of the head (COG). Chiropractic treatment consisted of thermal ultrasound therapy to the hypertonic muscles and high velocity, low amplitude spinal (upper thoracic and lumbar) manipulation to reduce intervertebral restriction. At the end of daily treatment for 5 consecutive days, the patient reported improvement in back and leg pain, walking ability, and insomnia symptoms. Intermittent motorized spinal traction (MID Series, WIZ Medical, Korea) was added to the second phase of treatment plan as spinal decompression. The patient had treatment sessions three times per week. At 11 weeks after initiating treatment, there was a significant improvement in various aspects of well-being, as evidenced by complete pain resolution (intensity from 8/10 to 0/10 on a numeric rating scale), a significant decrease in Parkinson’s Disease Questionnaire scores from 70 to 10 (PDQ-39 scores range from 0 to 100 with higher scores indicating worse level of disabling dyskinesia) (9), better postural stability (noticeable changes of X-ray parameters, as detailed on Figure 1B), and stable gait performance (accurate foot stride, regular heel strike and symmetric center of pressure crossover between strides depicted on gait cyclogram, Figure 2). There were no treatment-related adverse events reported. For a summary timeline events, please see Figure 3.
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). A written informed consent was obtained from the patient.
Discussion
Pain is a common non-motor symptom in PD that markedly impacts patients’ quality of life. PD-related pain is often experienced in the neck, upper back, and the legs, probably related to an exertion of the musculoskeletal system, such as head dropping, antecollis, thoracic kyphosis, camptocormia, truncal dystonia and gait disturbance (3). Pain may also originate from the normal age-related orthopedic comorbidities, such as degenerative joint changes, osteoporosis, and sarcopenia (8,10). However, approximately 8% to 40% of PD patients experienced pain predating the onset of motor features by years (10,11). To put pre-motor pain in context, pain perception is not simply a comorbidity of motor disability in early PD (8). Many areas and neurochemical systems of the central nervous system involved in the processing and modulation of pain are affected by PD (5). The pre-motor stage reflects early structural changes in the lower brainstem nuclei and peripheral nervous system, including the autonomic and enteric ganglia (10). Pathological findings of neuritic deposition of α-synuclein (a presynaptic neuronal protein) in the peripheral nerve fibers and skin denervation suggest that small-fiber neuropathy of idiopathic PD may contribute to the aberrant pain perception (12). In an autopsy study of an elderly cohort with Lewy body-related α-synucleinopathy, Lewy body was found in both the central and peripheral nervous systems, including the spinal cord, dorsal root ganglia, and preganglionic sympathetic nerves, of patients with PD (13). It is possible that the pathological processing of peripheral nerve degeneration in early PD, rather than simple pain perception, may progress to central nociceptive dysfunction (8).
The impacts of pain and motor disabilities may lead to a combination of physical dysfunction, depression, illness behavior and social interactions. Levodopa, a precursor of dopamine with antiparkinsonian properties, remains the single potent medication for motor symptoms of PD. Levodopa can normalize pain-induced activation in nociceptive pathways and raise pain thresholds in PD (6). For PD-related peripheral neuropathic and central pain that does not respond to anti-parkinsonism medications, co-analgesics against neuropathic pain, i.e., anticonvulsants and tricyclic antidepressants, are worth trying (8). The evidence for using analgesics or non-steroid anti-inflammatory drugs on pain relief in patients with PD is limited (14). Deep brain stimulation targeting the subthalamic nucleus and globus pallidus only has a negligible to mild effect on a specific type of PD-related pain (i.e., disabling trunk flexion due to camptocormia) (10). Traditionally, PD-related pain is categorized into five domains: musculoskeletal, dystonia-related, radicular/neuropathic, akathitic, and central pain (14,15). Of the diverse mechanisms of PD-related pain, musculoskeletal pain is the most common form, accounting for 40–90% of reported pain in PD patients (14). Complementary remedies for managing PD-related pain such as physiotherapeutic and chiropractic treatments are espoused, consisting of focused management of pain, strength and flexibility, and postural motor retraining (14,16).
Rigidity is a motor impairment with the strongest association with pain, while tremor does not show any significant association with pain in PD (5). It has been proposed that rigidity contributes to musculoskeletal pain due to its potential for altering normal posture, leading to stiffness, reduced flexibility and altered body mechanics (5). Rigidity, muscular tension and poor postural reflexes can also lead to balance deficits and postural instability (17). Pharmacological treatment or deep-brain stimulation for patients with PD is usually insufficient to control gait and balance adequately (18). Clinical trials have documented motor improvement in patients with PD following an 8-week physical rehabilitation (19), a 6-week osteopathic manipulation (17), or even a single-session of osteopathic treatment (20). Manual therapies, broadly speaking, are primarily used to treat musculoskeletal issues, with emphasis on a range of strategies to mobilize restricted structures and relieve neural compromise, and maximize the functions of contracted joints and affected muscles (21,22). As seen in the current case, reduced pain and improved gait stability observed after chiropractic care (Figure 2) were in line with previous reports documenting promise of chiropractic interventions for those with PD (23,24). It is speculated that pain relief following manipulative remedies may improve neural proprioception of muscles, motor functions, and postural balance (22).
Gait is one of the hallmarks for disease progression in patients with PD but there is no gold standard for tracking disease progress (24). Patients with PD with axial disability will typically adjust their movement pattern in order to move slowly and safely to avoid falls or injuries. With further disease progression, gait instability in the mediolateral plane may occur, as reflected in an abnormal tandem gait test (18). Instrumented treadmill analysis systems can implement and measure stepping paradigms during ambulation. The overlaid graphical display of dynamic center-of-pressure (COP) trajectory in patients with PD showed distorted butterfly wing feature (25) and reduced anteroposterior foot placement accuracy (26), as seen in Figure 2A. Dynamic gait cyclogram provides a new and intuitive way to analyze gait abnormalities of patients with PD (25). Changes of COP parameters significantly reflect patients’ gait patterns. The current findings and previous research (17) have demonstrated manipulative therapies can improve balance and motor function in individuals with PD. The limitations of this study were the small number of patients, the lack of a control group, and short follow-up period. The actual duration of sustaining improved outcomes following a course of manipulative intervention remains to be determined.
Conclusions
Presented is a case of PD in a patient exhibiting characteristic posture of PD, gait difficulty and low back pain, which were improved by 11 weeks of chiropractic manipulation. The current study may serve as an example of spinal manipulation showing the potential to address gait and posture problems associated with pain in a patient with PD.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/acr-21-27
Peer Review File: Available at https://dx.doi.org/10.21037/acr-21-27
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/acr-21-27). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and international research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Surmeier DJ. Determinants of dopaminergic neuron loss in Parkinson’s disease. FEBS J 2018;285:3657-68. [Crossref] [PubMed]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10)-WHO Version for; 2019-covid-expanded. Available online: https://icd.who.int/browse10/2019/en#/G20-G26. Last accessed Apr 15, 2021.
- Tai YC, Lin CH. An overview of pain in Parkinson's disease. Clinical Parkinsonism & Related Disorders 2020;2:1-8. [Crossref] [PubMed]
- Edinoff A, Sathivadivel N, McBride T, et al. Chronic Pain Treatment Strategies in Parkinson's Disease. Neurol Int 2020;12:61-76. [Crossref] [PubMed]
- Allen NE, Wong CM, Canning CG, et al. The Association between Parkinson's disease motor impairments and pain. Pain Med 2016;17:456-62. [Crossref] [PubMed]
- Yilmaz NH, Saricaoğlu M, Eser HY, et al. The Relationship between pain, and freezing of gait and falls in Parkinson's disease. Noro Psikiyatr Ars 2019;57:56-60. [PubMed]
- Zahoor I, Shafi A, Haq E. Pharmacological treatment of Parkinson’s Disease. In: Stoker TB, Greenland JC, editors. Parkinson’s Disease: Pathogenesis and Clinical Aspects. Brisbane (AU): Codon Publications, 2018. Chapter 7.
- Tseng MT, Lin CH. Pain in early-stage Parkinson’s disease: Implications from clinical features to pathophysiology. J Formosan Med Assoc 2017;116:571-81. [Crossref] [PubMed]
- Jesus-Ribeiro J, Vieira E, Ferreira P, et al. Reliability and Validity of 39-Item Parkinson's Disease Questionnaire and Parkinson's Disease Quality of Life Questionnaire. Acta Med Port 2017;30:395-401. [Crossref] [PubMed]
- Truini A, Frontoni M, Cruccu G. Parkinson's disease related pain: a review of recent findings. J Neurol 2013;260:330-4. [Crossref] [PubMed]
- Lin CH, Wu RM, Chang HY, et al. Preceding pain symptoms and Parkinson's disease: a nationwide population-based cohort study. Eur J Neurol 2013;20:1398-404. [Crossref] [PubMed]
- Donadio V, Incensi A, Leta V, et al. Skin nerve alpha-synuclein deposits: a biomarker for idiopathic Parkinson disease. Neurology 2014;82:1362-9. [Crossref] [PubMed]
- Sumikura H, Takao M, Hatsuta H, et al. Distribution of α-synuclesi in the spinal cord and dorsal root ganglia in an autopsy cohort of elderly persons. Acta Neuropathol Commun 2015;3:57. [Crossref] [PubMed]
- Skogar O, Lökk J. Pain management in patients with Parkinson's disease: challenges and solutions. J Multidiscip Healthc 2016;9:469-79. [Crossref] [PubMed]
- Young Blood MR, Ferro MM, Munhoz RP, et al. Classification and Characteristics of Pain Associated with Parkinson's Disease. Parkinsons Dis 2016;2016:6067132 [Crossref] [PubMed]
- Jacobs JV, Henry SM, Horak FB. What if low back pain is the most prevalent parkinsonism in the world? Front Neurol 2018;9:313. [Crossref] [PubMed]
- DiFrancisco-Donoghue J, Apoznanski T, de Vries K, et al. Osteopathic manipulation as a complementary approach to Parkinson's disease: A controlled pilot study. NeuroRehabilitation 2017;40:145-51. [Crossref] [PubMed]
- Raccagni C, Nonnekes J, Bloem BR., et al. Gait and postural disorders in parkinsonism: a clinical approach. J Neurol 2020;267:3169-76. [Crossref] [PubMed]
- Comella CL, Stebbins GT, Brown-Toms N, et al. Physical therapy and Parkinson's disease: a controlled clinical trial. Neurology 1994;44:376-8. [Crossref] [PubMed]
- Wells MR, Giantinoto S, D'Agate D, et al. Standard osteopathic manipulative treatment acutely improves gait performance in patients with Parkinson's disease. J Am Osteopath Assoc 1999;99:92-8. [PubMed]
- de Paula Vasconcelos LA. Parkinson’s disease rehabilitation: effectiveness approaches and new perspectives. In: Bernardo-Filho M, de Sá-Caputo D, Taiar R, editors. Physical Therapy Effectiveness. London, UK: IntechOpen, 2019.
- Chu ECP, Wong AYL, Lee LYK. Craniocervical instability associated with rheumatoid arthritis: a case report and brief review. AME Case Rep 2021;5:12. [Crossref] [PubMed]
- Anderson JM, Oakley PA, Harrison DE. Improving posture to reduce the symptoms of Parkinson's: a CBP® case report with a 21 month follow-up. J Phys Ther Sci 2019;31:153-8. [Crossref] [PubMed]
- Norkus S, Ryan S. The effect of spinal manipulation as complementary therapy for Parkinson's disease symptomatology: a literature review. Journal of Contemporary Chiropractic 2020;3:7-13.
- Shin C, Ahn TB. Asymmetric dynamic center-of-pressure in Parkinson’s disease. J Neurol Sci 2020;408:116559 [Crossref] [PubMed]
- Vitório R, Gobbi LTB, Lirani-Silva E, et al. Synchrony of gaze and stepping patterns in people with Parkinson’s disease. Behav Brain Res 2016;307:159-64. [Crossref] [PubMed]
Cite this article as: Chu ECP, Wong AYL, Lee LYK. Chiropractic care for low back pain, gait and posture in a patient with Parkinson’s disease: a case report and brief review. AME Case Rep 2021;5:34.