
Thymoma associated with severe pancytopenia and Good’s syndrome: case report
Introduction
Thymoma is known to have associated hematologic autoimmune and paraneoplastic syndromes. Approximately half of patients diagnosed with thymomas are found to have paraneoplastic syndromes, with myasthenia gravis being the most common and seen in up to 25–40% of patients with thymoma (1). Hematologic autoimmune manifestations are less common, and of these, pure red cell aplasia is the most common, seen in 2–5% of patients with thymoma. Pancytopenia and Good’s syndrome are both independently rare associated findings in patients with thymoma (2,3). Our patient was diagnosed with pancytopenia and thymoma upon presentation and found to have Good’s syndrome, in addition to pancytopenia, postoperatively. Surgery is the mainstay therapy for thymoma and paraneoplastic syndromes such as myasthenia gravis, but treatment guidelines for thymoma-associated hematologic autoimmune syndromes is less clear (4,5). Based on an extensive literature review, this patient’s presentation is extremely uncommon and has not been specifically reported prior to this.
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/acr-21-4).
Case presentation
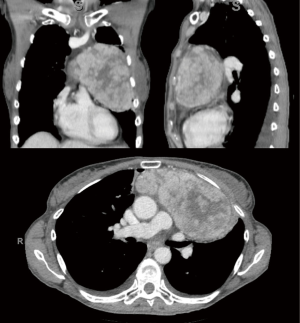
A previously healthy 60-year-old female presented to the emergency department with severe headaches, night sweats, low grade fevers, and ecchymoses. Her work up revealed an acute small parenchymal hemorrhage, severe pancytopenia, most notably thrombocytopenia with platelets of 2 (reference range, 140–400 K/uL) and absolute neutrophil count 0.2 (reference range, 1.8–7.9 K/uL), and a large anterior mediastinal mass with concern for thymoma (Figure 1). She underwent mediastinal mass and bone biopsy, which confirmed thymoma and empty marrow without hematopoiesis, respectively.
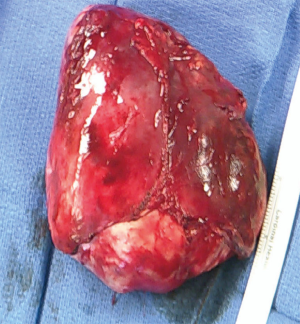
At time of consultation, the patient was transfusion-dependent due to her severe pancytopenia. Hematologic multidisciplinary discussion was held and it was decided that the patient would undergo immunosuppression with anti-thymocyte globulin/cyclosporine prior to resection. However, the patient was re-admitted to the hospital for pleural effusion and severe cellulitis. Bronchial washings revealed rhinovirus, enterovirus, and gram negative cocci. Hematology/oncology felt that the patient would not benefit from starting immunosuppressive therapy because it would exacerbate her infections by further compromising her immune function before improving her pancytopenia. After multidisciplinary discussion, it was decided to proceed with surgery first. She underwent an uneventful median sternotomy and total thymectomy (Figure 2). During dissection, the tumor was easily separated from neighboring structures and was able to be removed in its entirety. Final pathology demonstrated a 13 cm × 11 cm × 6.2 cm type A thymoma without invasion of surrounding structures. Pathologic stage pT1a, Nx.
Her cell lines remained unchanged postoperatively and she developed diarrhea and sepsis of unknown etiology. She remained on broad spectrum antimicrobials but was persistently febrile. She was found to be hypogammaglobulinemic, IgG 442 mg/dL (reference range, 600–1,600), and IgM 18 mg/dL (reference range, 30–190), one week postoperatively and was diagnosed with Good’s syndrome. She received intravenous immunoglobulin (IVIG) immediately thereafter. However, the day after receiving IVIG, she required cardiopulmonary resuscitation for aspiration. The patient was transferred to the ICU, but ultimately expired from multi-system organ failure.
Ethical approval was not obtained for this case report because the Institutional Review Board of Kaiser Permanente Northern California does not require approval for case reports. Written consent was not obtained from the patient or patient’s guardian/family for this case report. The Institutional Review Board of Kaiser Permanente Northern California does not require written consent for case reports. In addition, our decision to publish this paper was made posthumously. Due to the patient’s rather steep and rapid decline and death from time of diagnosis, we have decided that it would be intrusive to attempt to obtain written consent from her legal guardian. This decision was made also because we feel that the relevant data presented for the benefit of the medical community can largely be discussed while protecting the patient’s privacy. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013).
Discussion
Five to six percent of thymomas are associated with hematologic disorders (6). In our case study, the patient was diagnosed with not only pancytopenia, but also Good’s syndrome in correlation with thymoma, both of which are rare (2). Aplastic anemia is found in 0–1.4% of patients with thymoma, with an unknown incidence of pancytopenia. Although the patient was anemic (initial Hgb 6.1, ref 11.5–15 g/dL) throughout her admission and pure red cell aplasia is more common in patients with thymoma, the significant abnormalities in all of her cell lines and bone marrow biopsy with absent trilineage hematopoiesis led the authors to diagnose her with aplastic anemia instead of pure red cell aplasia. Outcomes for patients with aplastic anemia are poor and a cure has not been established. Thymectomy is the standard of care for patients with thymoma, but in patients with hematologic abnormalities, less than 30% remission is found in patients who undergo thymectomy (7). This is a possible reason that pancytopenia associated with thymoma is diagnosed both pre- and postoperatively. In our patient, she was symptomatic from her pancytopenia, presenting as hemorrhagic stroke, prior to her diagnosis of thymoma. Cyclosporine is the most frequent first line therapy for aplastic anemia, with a greater than 70% response rate, and treatment can be elevated to hematopoietic stem cell transplant if needed (8,9).
Similar to pancytopenia, Good’s syndrome is a rare condition, seen in 3–6% of patients with thymoma, and is defined by concurrent immunodeficiency. Official diagnostic criteria have not been defined, but patients typically have low to absent B cells, hypogammaglobulinemia, and cell-mediated immunity defects. Almost all patients have reduced immunoglobulins. The most common infection seen is recurrent sinopulmonary infections secondary to encapsulated organisms. About 50% of patients have diarrhea, with an unidentified pathogen in the majority of cases, which was similar to our patient’s postoperative diarrhea (10). It has been reported that up to 60% of patients with Good’s syndrome die from infection, and they have an overall mortality close to 45% (11).
In a review of fifty Good’s syndrome cases, all patients who underwent thymectomy continued to have opportunistic infections due to their diminished immunologic function. IVIG was given to 30 of these patients, with 23 responding favorably, albeit some were only transient responders (3). Thymoma is treated with thymectomy, but there has not been a standard protocol for the administration of IVIG or timing of surgery for the patients with concurrent hypogammaglobulinemia. A potential reason for this is that a significant number of patients are diagnosed after thymectomy, much like pancytopenia, and frequently years after resection (3,10,12).
In a case report where the patient was diagnosed with Good’s syndrome preoperatively, the patient was started on IVIG immediately postoperatively and was able to be discharged by postoperative day 8 (13). In our case, IVIG was administered on postoperative day 9, which is much earlier than most other case reports where Good’s syndrome was diagnosed postoperatively.
Conclusions
Good’s syndrome is a serious disorder and requires prompt diagnosis and treatment. Evaluating a patient’s immunoglobulin levels should be considered in a patient who is found to have a thymoma and a clinical course of multiple, persistent, or uncommon infections (14). Once a diagnosis is made, we advocate for considering early administration of IVIG. In addition, a diagnosis of pancytopenia in the setting of thymoma indicates an overall worse prognosis and multidisciplinary treatment approach should be utilized. Areas of continued study should include timing of resection in patients diagnosed with pancytopenia and Good’s syndrome and timing and indications for IVIG in patients with Good’s syndrome.
Acknowledgments
We would like to thank UCSF - East Bay and Kaiser Permanente Oakland Medical Center for their continued support and dedication to our education and training.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/acr-21-4
Peer Review File: Available at http://dx.doi.org/10.21037/acr-21-4
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/acr-21-4). DSH and JBV report that article processing charges was paid by the authors and reimbursed by the department of surgery at their institutions. SAW has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval was not obtained for this case report because the Institutional Review Board of Kaiser Permanente Northern California does not require approval for case reports. Written consent was not obtained from the patient or patient’s guardian/family for this case report. The Institutional Review Board of Kaiser Permanente Northern California does not require written consent for case reports. In addition, our decision to publish this paper was made posthumously. Due to the patient’s rather steep and rapid decline and death from time of diagnosis, we have decided that it would be intrusive to attempt to obtain written consent from her legal guardian. This decision was made also because we feel that the relevant data presented for the benefit of the medical community can largely be discussed while protecting the patient’s privacy. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhao J, Bhatnagar V, Ding L, et al. A systematic review of paraneoplastic syndromes associated with thymoma: Treatment modalities, recurrence, and outcomes in resected cases. J Thorac Cardiovasc Surg 2020;160:306-314.e14. [Crossref] [PubMed]
- Chintakuntlawar AV, Rizvi SA, Cassivi SD, et al. Thymoma-associated pancytopenia: immunosuppressive therapy is the cornerstone for durable hematological remission. Ann Hematol 2015;94:453-8. [Crossref] [PubMed]
- Tarr PE, Sneller MC, Mechanic LJ, et al. Infections in patients with immunodeficiency with thymoma (Good syndrome). Report of 5 cases and review of the literature. Medicine (Baltimore) 2001;80:123-33. [Crossref] [PubMed]
- Scorsetti M, Leo F, Trama A, et al. Thymoma and thymic carcinomas. Crit Rev Oncol Hematol 2016;99:332-50. [Crossref] [PubMed]
- Padda SK, Yao X, Antonicelli A, et al. Paraneoplastic Syndromes and Thymic Malignancies: An Examination of the International Thymic Malignancy Interest Group Retrospective Database. J Thorac Oncol 2018;13:436-46. [Crossref] [PubMed]
- De Giacomo T, Rendina EA, Venuta F, et al. Pancytopenia associated with thymoma resolving after thymectomy and immunosuppressive therapy. Case report. Scand J Thorac Cardiovasc Surg 1995;29:149-51. [Crossref] [PubMed]
- Liozon E, Touati M, Allegraud A, et al. Thymoma-associated pancytopenia: effectiveness of cyclosporine A. Ann Hematol 1998;77:175-8. [Crossref] [PubMed]
- Gendron N, de Fontbrune FS, Guyard A, et al. Aplastic anemia related to thymoma: a survey on behalf of the French reference center of aplastic anemia and a review of the literature. Haematologica 2020;105:e333-e336. [Crossref] [PubMed]
- Gaglia A, Bobota A, Pectasides E, et al. Successful treatment with cyclosporine of thymoma-related aplastic anemia. Anticancer Res 2007;27:3025-8. [PubMed]
- Kelleher P, Misbah SA. What is Good's syndrome? Immunological abnormalities in patients with thymoma. J Clin Pathol 2003;56:12-6. [Crossref] [PubMed]
- Kelesidis T, Yang O. Good's syndrome remains a mystery after 55 years: A systematic review of the scientific evidence. Clin Immunol 2010;135:347-63. [Crossref] [PubMed]
- Narahari NK, Gongati PK, Kakarla B, et al. Thymoma-associated immunodeficiency: a diagnostic challenge for the clinician. Asian Cardiovasc Thorac Ann 2017;25:146-9. [Crossref] [PubMed]
- DeBoard ZM, Taylor BJ. Good's Syndrome: Successful Management of Thymoma With Hypoimmunoglobulinemia. Ann Thorac Surg 2015;100:1903-5. [Crossref] [PubMed]
- Zaman M, Huissoon A, Buckland M, et al. Clinical and laboratory features of seventy-eight UK patients with Good's syndrome (thymoma and hypogammaglobulinaemia). Clin Exp Immunol 2019;195:132-8. [Crossref] [PubMed]
Cite this article as: Hsu DS, Wilde SA, Velotta JB. Thymoma associated with severe pancytopenia and Good’s syndrome: case report. AME Case Rep 2021;5:22.


