
Nontuberculous mycobacterial lung infections in patients with eating disorders: plausible mechanistic links in a case series
Introduction
Anorexia nervosa (AN) is a mental disorder characterized by severe restriction of food intake (AN-R) with or without binging and purging behaviors (AN-BP), resulting in dramatic weight loss, and a myriad of medical complications. Nontuberculous mycobacterial (NTM) lung infections in five women (ages 20–53 years old) with AN have been reported, with all as single case reports (1-6). Since NTM lung disease occurs much more commonly in elderly individuals, these cases in relatively younger individuals presented herein suggest that patients with very thin body habitus as seen in those with AN and other eating disorders are more vulnerable to NTM lung disease.
In the United States, NTM lung infection is caused primarily by Mycobacterium avium complex (MAC), Mycobacterium abscessus complex, and Mycobacterium kansasii (7). Lung disease due to NTM can be differentiated into two distinct forms although both may occur simultaneously in any person: the fibrocavitary form, most commonly occurring in older male smokers with emphysema, and the nodular bronchiectatic form, which appears most frequently in nonsmoking, thin, white, middle-aged to elderly women without a known history of underlying lung disease, but who invariably have bronchiectasis at the time NTM lung disease diagnosis is made (8).
We present three patients with AN-R or AN-BP associated with severe malnutrition, each of whom also manifested NTM lung disease. To the best of our knowledge, this is the largest series of such cases to date. All three were evaluated at “ACUTE”, an inpatient medical stabilization unit dedicated to the acute care of those with extreme forms of eating disorders. We also speculate whether occult and perhaps mild eating disorders may be present in a number of NTM lung disease patients—who are mostly women—since many have a life-long or nearly life-long thin body habitus with no other apparent risk factors for NTM lung disease.
We present the following case in accordance with the AME Case Series reporting checklist (available at http://dx.doi.org/10.21037/acr-20-101).
Case presentation
Case report #1
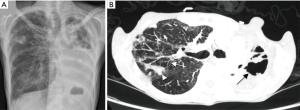
A 54-year-old woman with a longstanding history of AN-BP was admitted to ACUTE at Denver Health Medical Center at 72% of her ideal body weight (IBW) with a body mass index (BMI) of 15 kg/m2. She has a history of self-induced vomiting with progressive escalation the past several years, gastroparesis, mild esophageal dysmotility, dysphagia, and gastroesophageal reflux disease (GERD). While she had maintained her weight at approximately 75% of her IBW for a period of time, she developed more weight loss in the setting of new-onset respiratory symptoms found to be due to Mycobacterium abscessus lung disease. Antibiotics prescribed for the NTM lung disease were poorly tolerated, with side effects of nausea, vomiting and further weight loss. The patient was referred to our unit for weight restoration in anticipation of a planned right upper lobectomy. Outside hospital work up revealed an esophageal pH monitoring showing severe distal esophageal acid exposure; a swallow study demonstrating moderate pharyngeal dysphagia and aspiration; and an esophagram revealing mild esophageal dysmotility. A chest computed tomography (CT) demonstrated a right apical cavity, right upper lobe nodularity, and bronchiectasis (Figure 1). A bronchoscopy was performed, and the bronchial washings were positive for M. abscessus. Initial laboratory studies were normal other than mild lymphopenia.

The patient received nutritional rehabilitation, medical stabilization, and behavioral therapy as well as speech therapy for her dysphagia. Based on drug susceptibility testing of her M. abscessus, she received clofazimine, inhaled amikacin, and intravenous imipenem-cilastatin. She was initiated on more aggressive treatment for the GERD with omeprazole 40 mg BID and famotidine 40 mg BID as well as mechanical measures to prevent reflux.
The patient required supplemental enteral nutrition through a nasogastric tube due to her catabolic state and high caloric requirements for weight gain. Her nutrition plan at discharge was based on a 5,400-kcal/day diet to sustain her ongoing weight restoration. Upon discharge, she was at 85% of her IBW. She was transferred to a residential eating disorder center for further weight restoration before her planned partial lung resection, which was ultimately successfully performed when she was fully weight restored.
Case report #2
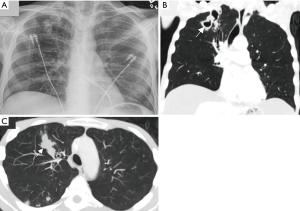
A 41-year-old woman with known MAC lung disease presented with a BMI of 11.1 kg/m2 (53% of IBW) for weight restoration in preparation for a planned left pneumonectomy. Her past medical history was also significant for remote Hodgkin’s lymphoma stage 4B with pulmonary involvement, treated with chemotherapy, local radiotherapy, and autologous stem cell transplantation. At age 33, she was diagnosed with NTM lung disease and was begun on daily azithromycin, ethambutol and rifampin with later addition of moxifloxacin and streptomycin. She underwent left lower lobectomy at age 34, and all antibiotics were stopped at age 35. Barrett’s esophagus was diagnosed at age 36. However, she was noted to have a recurrence of pulmonary MAC with cavitary disease at age 39 associated with worsening malnutrition (Figure 2). Therefore, azithromycin, ethambutol and rifampin were resumed, although she was frequently non-adherent.
While she denied any eating disorder symptoms including a drive for weight loss, body dysmorphia, or purging behaviors, she experienced emesis with increased oral intake. Upon admission to ACUTE, she was treated for presumed gastroparesis secondary to her malnutrition, and she underwent percutaneous endoscopic gastrostomy placement to supplement her oral intake given the high caloric diet required for her weight restoration. While it was the opinion of her providers that the emesis had a volitional component consistent with AN-BP, she was ultimately diagnosed with avoidant restrictive food intake disorder (ARFID), an eating disorder characterized by highly selective eating habits, disturbed feeding patterns, or both resulting in significant nutrition and energy deficiencies similar to what is observed in AN patients. The patient was discharged at 83.1% of her IBW with a 5,700-kcal/day nutritional plan.
Case report #3
A 41-year-old man with a past medical history of severe protein-caloric malnutrition, generalized anxiety disorder, and obsessive-compulsive disorder, was admitted to ACUTE for severe protein-calorie malnutrition complicated by hypoglycemia, various electrolyte and liver function test abnormalities, anemia and mild leukopenia. On presentation, his BMI was 16 kg/m2 (73% of his IBW); however, this was likely overestimated due to the presence of severe anasarca. He reported a 2-year history of weight loss that was not explained by any condition other than self-starvation. The patient ascribed his caloric restriction to his desire to stay healthy but also to symptoms of esophagitis and subjective dysphagia. Several weeks before admission, he developed progressive lower extremity edema unresponsive to increasing doses of furosemide. Three months before, the patient presented with odynophagia and oral leukoplakia and was diagnosed with oropharyngeal candidiasis and presumed Candida esophagitis for which he received a full course of fluconazole. Over the past several years, he had been noted to have pancytopenia occasionally requiring red blood cell transfusions. A bone marrow biopsy in January 2017 revealed a normocellular bone marrow with severe fat atrophy possibly related to severe malnutrition. A chest X-ray obtained during his admission to ACUTE revealed multiple right upper lobe nodular lesions, some of which appeared cavitary. A chest CT scan confirmed thick-walled cavitary lesions in the right upper lobe as well as bronchiectasis and severe panacinar emphysema (Figure 3). Acid-fast bacillus (AFB) staining of the sputum was positive, and cultures were positive for MAC. Since he had no history of purging, aspiration as a cause of bronchiectasis was not suspected. The white blood cell count, IgG levels, alpha-1 antitrypsin level and phenotype, rheumatoid factor, cyclic citrullinated peptide, Sjogren’s antibodies (anti-SS-A/anti-SS-B), Aspergillus antibodies, sweat chloride, urine histoplasma antigen, and serum Coccidioides antibody tests were all negative or within normal limits. An echocardiogram revealed a normal left ventricular systolic function, mild tricuspid regurgitation, normal pulmonary artery systolic pressures, and a small pericardial effusion. The cause of the patient’s anasarca was ultimately attributed to hypoalbuminemia and severe malnutrition. The patient’s MAC infection was initially treated with azithromycin, ethambutol, and rifampin. A repeat chest CT before discharge revealed that the cavitary nodules in the right upper lobe and superior segment of the right lower lobe had almost entirely resolved.
He was ultimately diagnosed with AN-R and received supportive psychotherapy, psychiatric consultation, and nutritional rehabilitation. He was able to reach 85% of his IBW by the time he was discharged from the hospital.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients.
Discussion
These three relatively young patients with caloric-restrictive eating disorders demonstrate that low body weight is a plausible risk factor for NTM lung disease since, except for the cystic fibrosis population, lung diseases attributed to these environmental organisms typically occur in older individuals. Nodular bronchiectasis due to NTM infection was first described predominately in older women without a preexisting illness (9).
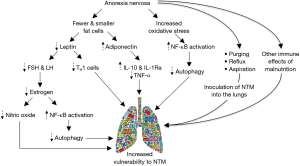
Pre-existing bronchiectasis and emphysema are the most prevalent predisposing conditions for NTM lung disease. Bronchiectasis may be due to prior suboptimal treated infections such as tuberculosis but may also be seen in the setting of cystic fibrosis, alpha-1-antitrypsin deficiency, primary ciliary dyskinesia, and connective tissue disorders like Sjogren’s syndrome. Furthermore, occult or symptomatic GERD—shown to be increased in patients with NTM lung disease—may exacerbate bronchiectasis as well as provide a mechanism by which NTM inoculate the lungs (Figure 4) (10-13). Interestingly, all our patients had various gastrointestinal disorders including dysphagia, GERD, emesis (volitional or non-volitional), and/or gastroparesis.
It has been observed that not an insignificant number of patients with NTM lung disease without known underlying host risk factors possess Marfanoid features such as life-long slender body habitus and thoracic cage abnormalities such as pectus excavatum and scoliosis (14-20). This observation has been substantiated by the documentation of reduced baseline BMI or body fat in pulmonary NTM patients (14,21-23). Two of the three patients with NTM lung disease in this case series are middle-aged white women with AN-R or AN-BP, and BMI between 10 and 16 kg/m2.
One mechanism by which slender individuals with low body fat content may be predisposed to NTM infections is their relative deficiency of leptin, an adipokine produced by fat cells whose canonical function is that of a satiety hormone (24). However, leptin has a number of immunomodulatory functions that can enhance host-immunity against NTM, including the differentiation of uncommitted T0 cells toward the TH1 interferon-gamma (IFN-γ)-producing phenotype (Figure 4) (19). Indeed, leptin-deficient mice are more susceptible to experimental M. abscessus lung infection (25). In addition, pulmonary NTM patients have been found to have reduced serum leptin levels (26) or a loss in the normal direct relationship between percent body fat and serum leptin concentration (27).
Very thin individuals with secondary leptin deficiency are prone to develop hypothalamic amenorrhea. The reason is that normally leptin induces the expression of follicle stimulating hormone (FSH) and luteinizing hormone (LH). Could disruption of this FSH/LH-estrogen axis due to leptin deficiency also help explain why such thin individuals are predisposed to NTM infections? Indeed, the nodular bronchiectasis form of NTM lung disease are disproportionately more common in post-menopausal women, accounting for 65–85% of the cases (11,28-31), suggesting that estrogen in women (and possibly androgen in men) may play a host-protective role. This notion is supported by reports of MAC infections in those with untreated panhypopituitarism (32).
Experimentally, binding of estrogen to estrogen receptors on macrophages has been shown to augment both phagocytic function and Fcγ receptor expression (33). Furthermore, estrogen is known to activate endothelial nitric oxide synthase (eNOS) (34), an enzyme that is also present on airway epithelial cells and that catalyzes the production of nitric oxide (NO), a free radical known to have anti-mycobacterial properties. While still early in its development as an anti-mycobacterial agent, inhalation of exogenous NO holds some promise as adjunctive treatment for NTM lung disease (35-37). This “estrogen-deficient hypothesis” of NTM susceptibility is corroborated by an in vivo study showing that oophorectomized mice were more susceptible to pulmonary MAC than mice with intact ovaries; furthermore, reconstitution with exogenous estrogen increased NO production and restored the ability of the oophorectomized mice to control the infection better, similar to that seen in the control mice (38).
Another plausible mechanism by which estrogen protects against NTM is the ability of estradiol to inhibit activation of nuclear factor-kappa B (NF-κB) in macrophages (34), and we have shown that NF-κB inhibition augments autophagy in macrophages, a known killing mechanism against mycobacteria (Figure 4) (39).
Thus, we believe that our three subjects with eating disorders have at least two clinical conditions that left them vulnerable to NTM lung disease: gastroesophageal disorders ± volitional or non-volitional emesis and very thin body habitus. The gastroesophageal disorder provided a mechanism by which NTM may be inoculated into the lungs through symptomatic or silent aspiration and the thin body habitus likely impaired their immunity against the opportunistic environmental organisms with relative low virulence through reduction in leptin and possibly other mechanisms such as secondary estrogen (or testosterone) deficiency. All three patients presented with nodular bronchiectasis with cavitary disease, the latter a sign of more severe NTM lung disease. Further prospective investigation is needed to determine whether there exists any causation effect between the purging behaviors of eating disorders and NTM lung infections, and whether reversing the complications of eating disorders improves or prevents the NTM lung infections.
Conclusions
The recognition of factors potentially associated with the increased susceptibility to developing NTM lung infections in patients with eating disorders is important to ensure earlier diagnosis and more rapid intervention to prevent disease progression and to exhort treatment of a covert or overt eating disorder. The well-described unique body habitus in some patients with NTM lung disease may indeed be a surrogate for an occult and underlying eating disorder. Whether the thin body habitus in patients with NTM lung disease is contributed by an underlying eating disorder or not, it remains to be determined whether effective nutritional repletion can positively affect the outcome of NTM lung disease and/or reduce the high rate of recurrence.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at http://dx.doi.org/10.21037/acr-20-101
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/acr-20-101). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Ethics Approval and Consent to Participate: GM/Cayuse# 19-0165, COMIRB# 19-0455. P.I. Daniela E. Grayeb, MD, P.I. institution DHHA. Written informed consent was obtained from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brown GR. Anorexia nervosa complicated by Mycobacterium xenopi pulmonary infection. J Nerv Ment Dis 1987;175:629-32. [Crossref] [PubMed]
- Hotta M, Minami Y, Itoda I, et al. A young female patient with anorexia nervosa complicated by Mycobacterium szulgai pulmonary infection. Int J Eat Disord 2004;35:115-9. [Crossref] [PubMed]
- Walsh TL, Baca V, Stalling SS, et al. Mycobacterium avium-intracellulare pulmonary infection complicated by cutaneous leukocytoclastic vasculitis in a woman with anorexia nervosa. Infection 2014;42:559-63. [Crossref] [PubMed]
- Cosson MA, Bertrand JB, Martin C, et al. Temporal interferon-gamma release response to Mycobacterium kansasii infection in an anorexia nervosa patient. J Med Microbiol 2012;61:1617-20. [Crossref] [PubMed]
- Tenholder MF, Pike JD. Effect of anorexia nervosa on pulmonary immunocompetence. South Med J 1991;84:1188-91. [Crossref] [PubMed]
- Portillo K, Morera J. Nutritional status and eating disorders: neglected risks factor for nontuberculous mycobacterial lung disease? Med Hypotheses 2012;78:39-41. [Crossref] [PubMed]
- McShane PJ, Glassroth J. Pulmonary disease due to nontuberculous mycobacteria. Current state and insights. Chest 2015;148:1517-27. [Crossref] [PubMed]
- Mirsaeidi M, Hadid W, Ericsoussi B, et al. Non-tuberculous mycobacterial disease is common in patients with non-cystic fibrosis bronchiectasis. Int J Infect Dis 2013;17:e1000-4. [Crossref] [PubMed]
- Prince DS, Peterson DD, Steiner RM, et al. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med 1989;321:863-8. [Crossref] [PubMed]
- Colombo RE, Hill SC, Claypool RJ, et al. Familial clustering of pulmonary nontuberculous mycobacterial disease. Chest 2010;137:629-34. [Crossref] [PubMed]
- Griffith DE, Girard WM, Wallace RJ Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis 1993;147:1271-8. [Crossref] [PubMed]
- Thomson RM, Armstrong JG, Looke DF. Gastroesophageal reflux disease, acid suppression and Mycobacterium avium complex pulmonary disease. Chest 2007;131:1166. [Crossref] [PubMed]
- Koh WJ, Lee JH, et al. Prevalence of gastroesophageal reflux in patients with NTM lung disease. Chest 2007;131:1825-30. [Crossref] [PubMed]
- Chan ED, Iseman MD. Slender, older women appear to be more susceptible to nontuberculous mycobacterial lung disease. Gend Med 2010;7:5-18. [Crossref] [PubMed]
- Guide SV, Holland SM. Host susceptibility factors in mycobacterial infection: Genetics and body morphotype. Infect Dis Clin North Am 2002;16:163-86. [Crossref] [PubMed]
- Iseman MD. The Theodore E. Woodward Award. Mycobacterium avium and slender women: an unrequited affair. Trans Am Clin Climatol Assoc 1998;109:199-202; discussion 203-4. [PubMed]
- Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis 1991;144:914-6. [Crossref] [PubMed]
- Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008;178:1066-74. [Crossref] [PubMed]
- Tasaka S, Hasegawa N, Nishimura T, et al. Elevated serum adiponectin level in patients with Mycobacterium avium-intracellulare complex pulmonary disease. Respiration 2010;79:383-7. [Crossref] [PubMed]
- Holt MR, Kasperbauer SH, Koelsch TL, et al. Similar characteristics of nontuberculous mycobacterial lung disease in men and women. Eur Respir J 2019;54:1900252. [Crossref] [PubMed]
- Lee SJ, Ryu YJ, Lee JH, et al. The impact of low subcutaneous fat in patients with nontuberculous mycobacterial lung disease. Lung 2014;192:395-401. [Crossref] [PubMed]
- Hayashi M, Takayanagi N, Kanauchi T, et al. Prognostic factors of 634 HIV-negative patients with Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2012;185:575-83. [Crossref] [PubMed]
- Lord GM, Matarese G, Howard JK, et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998;394:897-901. [Crossref] [PubMed]
- Ordway D, Henao-Tamayo M, Smith E, et al. Animal model of Mycobacterium abscessus lung infection. J Leukoc Biol 2008;83:1502-11. [Crossref] [PubMed]
- Chan ED, Kaminska AM, Gill W, et al. Alpha-1-antitrypsin (AAT) anomalies are associated with lung disease due to rapidly growing mycobacteria and AAT inhibits Mycobacterium abscessus infection of macophages. Scand J Infect Dis 2007;39:690-6. [Crossref] [PubMed]
- Daley CL, Griffith DE. Pulmonary disease caused by rapidly growing mycobacteria. Clin Chest Med 2002;23:623-32. [Crossref] [PubMed]
- Kartalija M, Ovrutsky AR, Bryan CL, et al. Patients with nontuberculous mycobacterial lung disease exhibit a unique body and immune phenotypes. Am J Respir Crit Care Med 2013;187:197. [Crossref] [PubMed]
- Field SK, Cowie RL. Lung disease due to more common nontuberculous mycobacteria. Chest 2006;129:1653-72. [Crossref] [PubMed]
- Okumura M, Iwai K, Ogata H, et al. Clinical factors on cavitary and nodular bronchiectatic types in pulmonary Mycobacterium avium complex disease. Intern Med 2008;47:1465-72. [Crossref] [PubMed]
- Koh WJ, Kwon OJ. Mycobacterium avium complex lung disease and panhypopituitarism. Mayo Clin Proc 2005;80:961-2. [Crossref] [PubMed]
- Chalermskulrat W, Gilbey JG, Donohue JF. Nontuberculous mycobacteria in women, young and old. Clin Chest Med 2002;23:675-86. [Crossref] [PubMed]
- Tsuyuguchi K, Suzuki K, Matsumoto H, et al. Effect of oestrogen on Mycobacterium avium complex pulmonary infection in mice. Clin Exp Immunol 2001;123:428-34. [Crossref] [PubMed]
- Deshpande R, Khalili H, Pergolizzi RG, et al. Estradiol down-regulates LPS-induced cytokine production and NFkB activation in murine macrophages. Am J Reprod Immunol 1997;38:46-54. [Crossref] [PubMed]
- Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev 2002;23:665-86. [Crossref] [PubMed]
- Bentur L, Masarweh K, Livnat-Levanon G, et al. Nitric oxide inhalations in CF patients infected with Mycobacterium abscessus complex: A prospective, open-labeled, multi-center pilot study. Am J Respir Crit Care Med 2018;201:A5919.
- Bogdanovski K, Ghaffari A, da Silva JL, et al. High-dose inhaled nitric oxide as a potential therapy against Mycobacterium abscessus lung infection in cystic fibrosis. Pediatr Pulmonol 2018;53:99.
- Miller CC, McMullin B, Martins J, et al. Inhaled gaseous nitric oxide as a treatment for nontuberculous mycobacteria lung infection study. Pediatr Pulmonol 2018;53:88.
- Bai X, Feldman NE, Chmura K, et al. Inhibition of nuclear factor-kappa B activation decreases survival of Mycobacterium tuberculosis in human macrophages. PLoS One 2013;8:e61925. [Crossref] [PubMed]
- Yaacoby-Bianu K, Gur M, Toukan Y, et al. Compassionate Nitric Oxide Adjuvant Treatment of Persistent Mycobacterium Infection in Cystic Fibrosis Patients. Pediatr Infect Dis J 2018;37:336-8. [Crossref] [PubMed]
Cite this article as: Grayeb DE, Chan ED, Swanson LM, Gibson DG, Mehler PS. Nontuberculous mycobacterial lung infections in patients with eating disorders: plausible mechanistic links in a case series. AME Case Rep 2021;5:9.


