
Tyrosine kinase inhibitor resistance: a case report on chronic myeloid leukemia and Gilbert’s syndrome
Introduction
Chronic myeloid leukemia (CML) is a clonal hematological malignancy, diagnosed by the detection of translocation between chromosomes 9 and 22 [t(Jen9;22) (q34.1;q11.2)] and, consequently, fusion between transcripts BCR and ABL (1), characterizing the Philadelphia chromosome. In order to distinguish the choric phase (CP) from accelerated phase (AP) and blastic phase (BP), other tests are required, as bone marrow aspirate, for morphology characterization and blast cells/basophils detection (2).
The gold standard treatment for CML requires a targeted therapy with tyrosine kinase inhibitors (TKI) aiming at the BCR-ABL1 kinase, which, by activating cellular signalling pathways and transcription, leads to cell proliferation, cell survival and apoptosis prevention (3). Protein tyrosine kinases (PTK) participate in molecular processes, involving growth factors, cytokines and hormones production and secretion that affect cell growth, stromal growth, angiogenesis, and tissue invasion. Various mechanisms are known to inhibit these molecules by multiple signalling pathways, as competition with ATP or other substrates (4).
Abnormal PTK expression can lead to not only cell invasion, metastasis, and tumor neovascularization, in cases of solid tumors, but also chemotherapy resistance, in cases of hematological malignancies. Besides TKIs have revolutionized cancer treatment, patients develop resistance, frequently and targeted therapy usually lasts only 5 to 9 months (5).
Mutations in the BCR-ABL kinase domain is the most prevailing resistance mechanism and are able to cause the interruption of critical contact points between the PTK and the drug. Another aspect of this process is the induction of conformational changes in the protein, inhibiting TKIs binding, specially Imatinib. In order to reduce resistance levels, novel TKIs have been developed, as Dasatinib, which inhibits almost all mutations in kinase domain that display resistance toward Imatinib and enables the patient to achieve faster and more efficient response to treatment (6).
However, some TKIs are associated to UGT1A1 polymorphism and its use in treatment may lead to the development of hyperbilirubinemia, a very common condition found in patients with Gilbert’s syndrome. Gilbert’s syndrome is a hereditary disease that can cause hyperbilirubinemia induced by the glucuronidation through reduced expression of UGT1A1 gene, which encodes UDP-glucuronosyltransferase (UGT), a hepatic enzyme that is able to glucuronide bilirubin. Mutations in the UGT1A1 generate abnormal unconjugation of bilirubinemia, establishing the hyperbilirubinemia (7). Additionally, polymorphisms in the UGT1A1 gene is associated to induced hyperbilirubinemia by TKIs in CML patients. Gilbert’s syndrome is mainly associated to UGT1A1*28 variant, which is characterized by the insertion of thymine-adenine (TA) in UGT promoter region, forming as the 7/7 polymorphism. Individuals that carry the homozygous form can develop higher levels of serum bilirubin than the heterozygous (8).
In light of the foregoing, our aim is to report the case of a CML patient that was treated with a TKI called Imatinib and developed resistance to this chemotherapeutic compound. During this process, the patient was diagnosed with homozygous (UGT1A1*28) Gilbert’s syndrome, while no alterations in bilirubin levels was observed. Therefore, we present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/acr-20-107).
Case presentation
A 34-year-old female was diagnosed with CML due to the presence of the Philadelphia chromosome. She reported nausea, diarrhea, flatulence, and myalgia. On physical examination, spleen and liver were palpable, indicating hepatosplenomegaly. Laboratory findings of peripheral blood showed leukocytosis (165,190/mm3), 6% of blasts and leukemic reaction as a result of an unstaged left shift, eosinophilia, basophilia, lymphocytosis and monocytosis. The bone marrow biopsy showed hypercellularity by granulocytic series with moderate maturation delay. Immediately, the patient started chemotherapy using a TKI, the Imatinib (300 mg/day). One year after the beginning of treatment, due to partial response to Imatinib, the therapy was changed to another TKI, Nilotinib (200 mg/day). A complete response to chemotherapy was observed since BCR-ABL translocation quantitative exams revealed a decrease from 211.7% (p210) to 0% of the chimeric gene. Despite the slight hyperbilirubinemia (2.3 mg/dL), a genetic study by polymerase chain reaction (PCR), followed by restriction fragment length polymorphism (RFLP) analysis, suggested the presence of the Ta7/TA7 allele of UGT1A1 gene, indicating positivity for Gilbert’s Syndrome (Table 1).
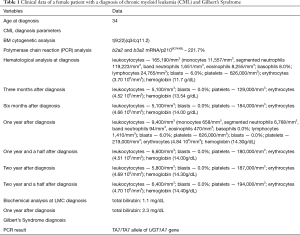
Full table
Two years after Gilbert’s syndrome diagnosis and treatment with Nilotinib, real-time PCR technique showed a drop in 3log and the translocation test of the BCR-ABL gene was positive for the isoforms b2a2 and b3a2 mRNA, suggesting that the patient went into a partial molecular remission of CML, still demanding continuance of chemotherapy. Nowadays, the patient continues to be treated with Nilotinib drug and has related no symptoms (Figure 1).

All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
CML is a hematological neoplasm associated with the presence of the Philadelphia chromosome, resulted from a translocation that results in the formation of the BCR-ABL oncogene, which encodes a protein with tyrosine kinase (TK) activity (9). The target therapy that revolutionized the treatment of CML was made by the TKI called Imatinib. This drug is able to significantly improve the prognosis and the evolution of patients with CML. However, some patients have mechanisms of resistance or intolerance, which prevent the eradication of CML (10) and could act on other enzymes, causing syndromes related to the metabolism, particularly in liver (11).
We reported a case of a patient that was diagnosed with CML by bone marrow cytogenetics analysis with the Philadelphia chromosome (t(9;22)(q34;q11.2)) and confirmation by PCR analysis (b2a2 and b3a2 mRNA and p210BCR/ABL – 221.7%). At the diagnosis, bilirubin level was 1.0mg/dL and Gilbert’s Syndrome polymorphism was not investigated due to the absence of hyperbilirubinemia. After diagnosis of CML, the patient was initially treated with Imatinib, and presented response to treatment indicated by a decrease in the leukocytes levels. However, response to Imatinib was lost over the evolution of treatment, requiring an alternative therapy and, therefore, Nilotinib, a second generation of tyrosine kinase drug, was prescribed. A reduction in the BCR-ABL translocation was detected with Nilotinib treatment while a slight hyperbilirubinemia (1.6 mg/dL) was observed. In this way, a PCR analysis for UGT1A1 gene polymorphism was done, revealing the presence of Gilbert’s Syndrome (TA7/TA7 allele presence of UGT1A1 gene). Gilbert’s Syndrome is a common liver disease caused by a mutation on the UGT1A1 gene. This gene normally enables the synthesis of a liver enzyme, which acts on hepatic phase II metabolism, converting unconjugated bilirubin into conjugated bilirubin (12). In the Gilbert’s syndrome, the process of bilirubin occur inappropriately due to deficient glucuronidation, causing elevation in the levels of bilirubin and a consequent mild hyperbilirubinemia (13). Gilbert’s syndrome patients could present serum bilirubin levels ranging from 0.6 to 3.0 mg/dL. Usually, individuals with Gilbert’s Syndrome are asymptomatic or exhibit mild yellowing of the skin, mucous membranes, and whites of the eyes. Furthermore, TKIs, as Nilotinib, are also a potent non-competitive inhibitors of the enzyme UGT1A1, which can lead to the worsening of Gilbert’s Syndrome (14). Interestingly, although a gene alteration was found and two coexisting conditions that predisposes to hyperbilirubinemia occurred, the patient did not present symptoms of jaundice.
This report demonstrates a case of a CML patient treated with two different TKIs that was subsequently diagnosed with Gilbert’s syndrome and it highlights the importance of having genetic investigations in cancer patients, in order to identify secondary diseases that could worsen the course of treatment. Adjusting chemotherapy is equally important to minimize resistance and other side effects, as hyperbilirubinemia. In this case, a biochemical panel should be periodically done to identify possible bilirubin levels alterations and avoid hepatic overload.
This thorough and deep investigation and exploration provided detailed (rich qualitative) information about a specific and rare case of two coexisting conditions that could cause hyperbilirubinemia, in absence of bilirubin alterations. Moreover, it highlights the importance of having genetic investigation in cancer patients, in order to identify secondary diseases that could worsen the course of treatment, providing insights for further researches. However, there are some limitations, as the difficulty to replicate and the conclusions drawn from a particular case may not be transferable to other settings.
Acknowledgments
The authors would like to thank Hospital Universitário São Francisco (HUSF) for their medical assistance. We also thank Universidade São Franscisco for academic assistance.
Funding: This study was funded by grants from the Universidade São Francisco through the Programa de Iniciação Científica, Iniciação Tecnológica e Extensão (PICITExt) da Universidade São Francisco – USF (Edital Proepe 2/2016 and 2/2017).
Footnote
Reporting checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/acr-20-107
Peer Review File: Available at http://dx.doi.org/10.21037/acr-20-107
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/acr-20-107). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient. This study was approved by the Human Ethics Committee of the University under number 69061417.2.0000.5514, 08037719.2.0000.5514 and 19295019.6.0000.5514.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aladağ E, Haznedaroğlu İC. Current perspectives for the treatment of chronic myeloid leukemia. Turk J Med Sci 2019;49:1-10. [PubMed]
- Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020;34:966-84. [Crossref] [PubMed]
- Özgür Yurttaş N, Eşkazan AE. Novel therapeutic approaches in chronic myeloid leukemia. Leuk Res 2020;91:106337. [Crossref] [PubMed]
- Bhullar KS, Lagarón NO, McGowan EM, et al. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer 2018;17:48. [Crossref] [PubMed]
- Jiao Q, Bi L, Ren Y, Song S, et al. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol Cancer 2018;17:36. [Crossref] [PubMed]
- Rossari F, Minutolo F, Orciuolo E. Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy. J Hematol Oncol 2018;11:84. [Crossref] [PubMed]
- Mi XX, Yan J, Ma XJ, et al. Analysis of the UGT1A1 Genotype in Hyperbilirubinemia Patients: Differences in Allele Frequency and Distribution. Biomed Res Int 2019;2019:6272174. [Crossref] [PubMed]
- Takano M, Sugiyama T. UGT1A1 polymorphisms in cancer: impact on irinotecan treatment. Pharmgenomics Pers Med 2017;10:61-8. [Crossref] [PubMed]
- Kang ZJ, Liu YF, Xu LZ, et al. The Philadelphia chromosome in leukemogenesis. Chin J Cancer 2016;35:48. [Crossref] [PubMed]
- Chopade P, Akard LP. Improving Outcomes in Chronic Myeloid Leukemia Over Time in the Era of Tyrosine Kinase Inhibitors. Clin Lymphoma Myeloma Leuk 2018;18:710-23. [Crossref] [PubMed]
- Saif MW, Smith MH, Maloney A, et al. Imatinib-induced hyperbilirubinemia with UGT1A1 (*28) promoter polymorphism: first case series in patients with gastrointestinal stromal tumor. Ann Gastroenterol 2016;29:551-6. [Crossref] [PubMed]
- Lv X, Xia Y, Finel M, et al. Recent progress and challenges in screening and characterization of UGT1A1 inhibitors. Acta Pharm Sin B 2019;9:258-78. [Crossref] [PubMed]
- Sun L, Li M, Zhang L, et al. Differences in UGT1A1 gene mutations and pathological liver changes between Chinese patients with Gilbert syndrome and Crigler-Najjar syndrome type II. Medicine (Baltimore) 2017;96:e8620. [Crossref] [PubMed]
- Fujita K, Sugiyama M, Akiyama Y, et al. The small-molecule tyrosine kinase inhibitor nilotinib is a potent noncompetitive inhibitor of the SN-38 glucuronidation by human UGT1A1. Cancer Chemother Pharmacol 2011;67:237-41. [Crossref] [PubMed]
Cite this article as: Bueno MLP, Roversi FM. Tyrosine kinase inhibitor resistance: a case report on chronic myeloid leukemia and Gilbert’s syndrome. AME Case Rep 2021;5:1.


